Fresh data confirm potential of CVac
The final data from the CAN-003 trial in ovarian cancer give a further indication of CVac's potential. The results in the second-line setting are even more impressive than the preliminary data previously reported. They justify the revised trial design for CAN-004b, which is now only recruiting patients with second-line ovarian cancer. More mature overall survival (OS) data from CAN-003 should be reported in Q414, which we expect will confirm the potential of CVac. We have increased our valuation to A$101m.
PFS benefit in second-line ovarian cancer confirmed
There was a trend towards an improvement in progression-free survival (PFS) in the Phase II CAN-003 trial in the CVac arm compared to placebo in the total patient population (median PFS: 12.9 vs 8.6 months, HR=0.72, n=56, first- and second-line ovarian cancer). There was no difference between the two trial arms in the first-line setting (HR=1.18, p=0.69). However, there was a statistically significant benefit observed in the second-line setting (median PFS of >12.9 vs 4.9 months, HR=0.32, p=0.04, n=20).
OS data is immature but already very promising
The OS data is still immature, but there appears to be a clear OS benefit in the second-line setting in patients receiving CVac (HR=0.17, p=0.07). In the first-line setting, there is no obvious difference. With immunotherapies, the improvement in OS is often greater than with PFS, so we remain confident that more mature OS data in Q414 will confirm that there is a benefit from CVac in the second-line setting, and it is possible that an improvement will be seen in the first-line setting.
CVac development firmly back on track
The final data from the CAN-003 trial supports the decision of Prima Biomed Ltd (ASX:PRR) to amend the CAN-004 trial and restrict recruitment to second-line ovarian cancer in CAN-004b. The trial, restarted in April, should be completed in mid-2015. The FDA has granted CVac Fast Track Designation, which will help to expedite the development process and highlights the clinical need in ovarian cancer.
Valuation: DCF valuation of A$101m
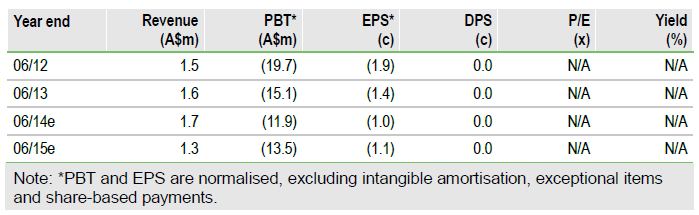
We increase our valuation by A$8m to A$101m (8.3c/share) after increasing the likelihood of success for CVac in ovarian cancer from 30% to 40% due to the impressive initial OS data. There are no changes to our estimates. The next catalysts for the shares are the start of the CAN-301 trial in pancreatic cancer in mid-2014 and additional OS data from CAN-003 in Q4 CY14.
To Read the Entire Report Please Click on the pdf File Below