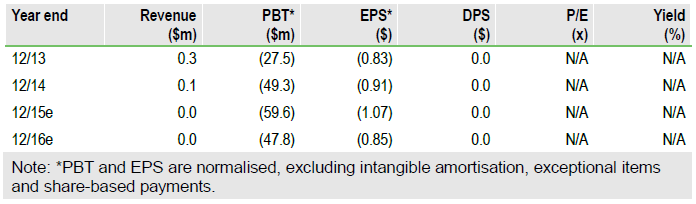
Cardiac safety data support investment case
CytRx released cardiotoxicity data and interim positive efficacy results from the Phase II GBM and combination Phase Ib aldoxorubicin studies. Our focus remains on the ongoing Phase III second-line soft tissue sarcoma (STS) trial, with data expected in H216. While our fundamental view on the drug’s potential has not changed, the supplied cardiotoxicity data supports its intended mechanism of action. That is, by acting as a safer form of doxorubicin, aldoxorubicin may displace it in STS and potentially other oncology indications. We have amended our valuation slightly, resulting in an rNPV of $381m (from $375m previously).
No cardiotoxicity in 200 evaluable patients to date
The newly-presented cardiac safety data from ASCO show that aldoxorubicin has not provoked cardiotoxicity in patients treated to date, including at cumulative doses well above recognised cardiac safety limits for doxorubicin. Doxorubicin causes toxicity in 6-20% of patients when the cumulative total dose is 500mg/m2; however, the majority of the 200 evaluable aldoxorubicin-treated patients received doses above 1,500mg/m2 without any clinically significant cardiotoxicity.
Signs of efficacy in GBM Phase IIb interim data
Interim data from 18 subjects with unresectable glioblastoma multiforme (GBM) showed that two subjects achieved a complete response (CR), although 14 patients discontinued therapy. CytRx believes that some of the discontinuations resulted from a mistaken assessment of tumour progression, when pseudo-progression (with tumour destruction) was occurring instead. CytRx will assess approaches to better differentiate these events in a future study, which could be a pivotal GBM trial that combines aldoxorubicin with bevacizumab. The firm and its investigators believe that aldoxorubicin can cross the blood-brain-barrier (BBB) to deliver treatment effects, justifying continued clinical development.
To Read the Entire Report Please Click on the pdf File Below