Methods
A suspension of pantoprazole 2 mg/mL was prepared by triturating 20 40-mg tablets of pantoprazole,a mixing with sterile water for irrigationb and sodium bicarbonate,c and diluting to a final volume of 400 mL with sterile water for irrigation (appendix). The suspension was transferred to six 3-oz amber polyethylene terephthalate bottles with childresistant caps,d a 3.75-mL sample was removed from each bottle and analyzed immediately after mixing, and the bottles were stored in the refrigerator (2-8 °C). At 8, 15, 22, 34, 49, and 62 days, each bottle was inverted and shaken manually to ensure a uniform suspension, and a 3.75-mL sample was removed for analysis. The pH of the suspension was measured initially and at each test interval.
Pantoprazole concentrations were measured by using a modified stabilityindicating high-performance liquid chromatographic (HPLC) method.[7,8,9] The HPLC system used has been described previously.[10] The reversephase columne was used at room temperature. The detector was set at a wavelength of 280 nm and a sensitivity of 0.01 absorption unit fullscale. The mobile phase, consisting of 40% (v/v) HPLC-grade acetonitrilef in 50 mM dibasic potassium phosphateg in HPLC-grade waterh (adjusted to pH 7.0 with phosphoric acidi), was passed through a 0.2-µm membrane filterj and degassed with helium. The flow rate of the mobile phase was 1.6 mL/min. The injection volume was 10 µL.
A stock solution (1 mg/mL) of the internal standard, ethylparaben,k was prepared by dissolving 200 mg in 80 mL of HPLC-grade methanoll and diluting to 200 mL with HPLC-grade water. The stock internal standard was stable for at least four months at room temperature (coefficient of variation, 3.2%). A separate solution of only the internal standard (20 µg/ mL) was injected at each test interval, with no change in peak appearance and no extraneous peaks observed. A stock standard solution (1 mg/mL) of pantoprazole was prepared by dissolving lyophilized pantoprazole sodium for injectionm in HPLC-grade water. Pure pantoprazole to be used as a reference standard could not be obtained from the manufacturer. Therefore, the injectable product was used to prepare the standards. According to the package insert,[2] it contains only pantoprazole sodium. The same lot of product was used as the standard throughout the study. Seven calibration standards were prepared by diluting the pantoprazole stock standard solution with HPLCgrade water to concentrations of 6, 9, 12, 15, 18, 21, and 24 µg/mL, each containing 20 µg of internal standard per milliliter. The standard calibration plots of pantoprazole peak area versus concentration and peak height versus concentration were linear (r2 = 0.9998 for both) and intersected the origin. The 15-µg/mL solution was used as the working standard solution. Both the stock and working standard solutions were prepared fresh at each test interval. The maximum interday and intraday coefficients of variation over 62 days were 1.0% and 0.9%, respectively.
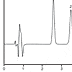
At each test interval, 3.75 mL of suspension from each bottle was pipetted into a separate 50-mL volumetric flask. About 25 mL of HPLCgrade water was added to each flask, and the flasks were placed on an orbital shaker for about 20 minutes. The contents of each flask were then diluted to volume with HPLC-grade water. A 5-mL portion of each diluted sample was further diluted to 50 mL with HPLC-grade water and 20 µg of internal standard per milliliter. Before injection into the HPLC system, each standard and sample solution was passed through a 0.2-µm membrane filter.n After each injection, the column effluent was monitored for four minutes. The retention times for pantoprazole and the internal standard were approximately 2.6 and 3.5 minutes, respectively (Figure 1).
Typical chromatogram of pantoprazole oral liquid stored at 2-8 °C. Peak 1 = pantoprazole (15 µg/mL), peak 2 = ethylparaben internal standard (20 µg/ml).
The HPLC assay for pantoprazole was demonstrated to be stability indicating. Two 40-mg pantoprazole tablets were triturated to a powder. Three 100-mg samples of the powder were mixed with 2.5 mL of 8.4% sodium bicarbonate in sterile water for irrigation. Two of the mixtures were adjusted to pH 0.1 and 12.9 with 10 N hydrochloric acid and 10 N sodium hydroxide, respectively. The third mixture was kept at the initial pH of 8.5. All three mixtures were placed in an oven at 60 °C for 20 hours. The pH of the mixtures was then adjusted back to approximately 8.5, and all three mixtures were diluted to a calculated pantoprazole concentration of 15 µg/mL, filtered, and analyzed. Complete degradation (100%) occurred at pH 0.1, and partial degradation of 14% and 4% occurred at pH 8.5 and 12.9, respectively. No interfering peaks were observed (Figure 2). Samples of tablet excipients not subjected to heating at 60 °C were tested by HPLC; none eluted with pantoprazole or the internal standard.
Chromatograms of pantoprazole after being stored at 60 °C for 20 hours at pH 8.5 (A), pH 0.1 (B), and pH 12.9 (C). Peaks 1-8 = unidentified degradation products, peak 9 = pantoprazole.
The stability of pantoprazole was assessed by evaluating the percentage of the initial concentration remaining at each test interval. A loss exceeding 10% of the initial concentration was considered to represent instability.
Am J Health Syst Pharm. 2002;59(10) © 2002 American Society of Health-System Pharmacists
Cite this: Stability of Pantoprazole in an Extemporaneously Compounded Oral Liquid - Medscape - May 15, 2002.







Comments