Results
A total of 251 citations were identified using the search strategy described above. Of these, 242 were excluded by abstract review alone as not being relevant. Included in the original analysis for the Cochrane Collaboration was a subgroup of three adult studies that are excluded here.[10,11,12] One trial in neonates that was excluded[13] in the original analysis because stridor was not assessed (and the specified primary outcome of that review was reintubation caused by stridor) is included here.
A total of six randomized trials were identified that addressed the effects of treatment with corticosteroids on reintubation or postextubation stridor in patients in the intensive care unit. Five studies involved dexamethasone treatment prophylactically before a patient's first elective extubation-three in neonates and two in older infants and children-and one study assessed the effect of dexamethasone on secondary extubation in pediatric patients already reintubated for stridor after a previous extubation attempt.[14]
Neonatal Prophylactic Studies (n = 3). Ferrara et al.[15] included 59 neonates intubated for ≤48 hrs, but they excluded infants intubated more than once. Couser et al.[16] selected 50 "high-risk" babies who underwent traumatic or multiple endotracheal intubations, or who were intubated at least 14 days. Courtney et al.[13] studied 51 infants of >1000 g in weight who were intubated between 3 and 30 days. Ferrara et al. used a single dose of 0.25 mg/kg intravenous dexamethasone 30 mins before extubation, Couser et al. gave a total of three doses (one 4 hrs before extubation, then every 8 hrs after extubation), and Courtney et al. gave three doses of 0.5 mg/kg intravenously every 8 hrs, with all doses given before elective extubation.
Pediatric Prophylactic Studies (n = 2). Anene et al.[17] studied 63 patients (age range, 1-59 months) in the pediatric intensive care unit who were intubated for >48 hrs, included patients with underlying airway abnormalities (e.g., subglottic stenosis, vocal cord paralysis), and only excluded patients with laryngotracheal infections or steroid use within 7 days of treatment. Tellez et al.[18] included 153 intubated patients (age ± sem of treated children, 2.5 ± 0.55 yrs; age of controls, 2.1 ± 0.4 yrs) in two pediatric intensive care units, excluding patients with primary upper airway infection, surgical trauma to the upper airway, or a history of previous upper airway obstruction. Both studies used 0.5 mg/kg dexamethasone (maximum, 10 mg), with the first dose 6-12 hrs before extubation, then every 6 hrs for six total doses.
The sole study designed to assess the effect of dexamethasone on the reintubation rate after an initial "failed" extubation (failure caused by postextubation stridor) studied 26 pediatric patients.[14] Dexamethasone (0.5 mg/kg) was given 6 hrs before the second extubation attempt and then at 6 and 12 hrs after extubation. This was the only study to use a scoring system for stridor, adapted from Leipzig et al.[19] Assessing audible stridor, cyanosis, sternal retractions, and respiratory and heart rates, scores could range from 0 to 11 (maximum).
There was no disagreement between reviewers with respect to quality assessment done independently. After discussion, there was also no disagreement regarding data extraction. Some of the information detailing methods of concealment and randomization were only obtained after direct contact with authors.
In Tellez et al.,[18] allocation concealment was uncertain (grade B). All others had adequate concealment (grade A). All studies were described as randomized. Appropriate methods of randomization were specified in all. All the investigations were described as double-blind, with adequate descriptions of control treatment. Tellez et al.[18] and Couser et al.[16] made no mention of withdrawals or dropouts. In the remainder of the studies, all postrandomization patients were accounted for. The pediatric studies were rated (according to the method of Jadad et al.) as a score of 3 (Tellez et al.), 5 (Anene et al.), and 5 (Harel et al.); the neonatal studies as 4 (Couser et al.) and 5 (Ferrara et al. and Courtney et al.).
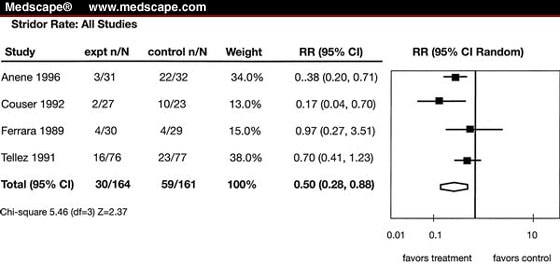
Combining neonatal and pediatric patients together showed a trend toward reduced reintubation rates without achieving statistical significance (total: n = 376, relative risk [RR] = 0.34, 95% confidence interval [CI] = 0.05-2.33) (Fig. 1). In the evaluation of stridor prevention, both groups demonstrated a significant reduction in stridor with corticosteroids (RR = 0.50, 95% CI = 0.28-0.88) (Fig. 2).
All studies: reduction in reintubation with prophylactic dexamethasone. RR, relative risk; CI, confidence interval; df, degrees of freedom.
All studies: reduction in stridor with prophylactic dexamethasone. RR, relative risk; CI, confidence interval; df, degrees of freedom.
In the neonatal studies, there was a trend toward a reduced rate of reintubation with prophylactic intervention (n = 160, RR = 0.2, 95% CI = 0.04-1.11). The trials also demonstrated a trend toward reduction in stridor prevalence with intervention in 109 patients (RR = 0.42, 95% CI = 0.07-2.32). There is heterogeneity in both outcomes between studies, however, suggesting perhaps that the multiple-dose strategy in the higher-risk patients of the Couser et al. and Courtney et al. studies is more effective than the single-dose regimen in lower-risk patients of the trial by Ferrara et al.. Other outcome measures sought, such as number of racemic epinephrine treatments or croup scores, were not reported in these studies.
In the two pediatric prophylaxis trials with 216 total patients, reintubation rates were not significantly reduced with intervention (RR = 0.49, 95% CI = 0.01-19.65). However, there was a significant reduction in postextubation stridor with intervention (RR = 0.53, 95% CI = 0.28-0.97). A trend toward a reduction in racemic epinephrine use for postextubation stridor was observed in these studies (RR = 0.38, 95% CI = 0.1-1.43). Once again, there is heterogeneity in all of these outcomes between trials, with the Anene et al. study demonstrating a significant reduction in reintubation rates, stridor, and racemic epinephrine use. The control rate of stridor and reintubation was notably higher in the trial by Anene et al., perhaps suggesting a higher-risk patient group. By description alone, both studies included similar patients and employed identical treatment regimens.
Harel et al.[14] was the sole study examining the effect of dexamethasone on postextubation stridor and reintubation in patients (children) already reintubated for postextubation airway obstruction. In these 33 patients, dexamethasone had no effect on postextubation stridor score (weighted mean difference, -1.1; 95% CI = -3.7 to 1.5). Although the RR reduction on reintubation rate was 45%, this did not achieve statistical significance (RR = 0.55, 95% CI = 0.17-1.78).
Few studies noted significant complications possibly attributable to steroid therapy, and these could not therefore be pooled. In Couser et al., 7 of 27 treated infants developed glucosuria, compared with 0 of 23 controls. Anene et al. noted one treated patient with gastrointestinal bleeding, and two patients-one treated and one control-developed hypertension.
Pediatr Crit Care Med. 2002;3(3) © 2002 Lippincott Williams & Wilkins
Cite this: Corticosteroids for the Prevention of Reintubation and Postextubation Stridor in Pediatric Patients: A Meta-Analysis - Medscape - Jul 01, 2002.









Comments