Deciding how to diagnose and treat prostate cancer has long been the subject of controversy and uncertainty. A prime example involves prostate-specific antigen (PSA) testing, a blood test for a telltale protein that can reveal cancer even when the patient has no symptoms. After its introduction in the early 1990s, PSA testing was widely adopted—millions of tests are done in the U.S. every year. In 2012, however, a government task force indicated that this test can lead to overtreatment of cancers that might have posed little danger to patients and so might have been best left alone.
While arguments for and against PSA testing continue to seesaw back and forth, the field has achieved a better grasp on what makes certain prostate cancers grow quickly, and those insights have paved the way for better patient prognoses at every stage of the disease, even for the most advanced cases. A prostate cancer specialist today has access to an enhanced tool set for treatment and can judge when measures can be safely deferred.
The importance of these advances cannot be overstated. Prostate cancer is still one of the most prevalent malignancies. Aside from some skin cancers, prostate cancers are the most common cancers among men in the U.S. Nearly 270,000 people in America will be diagnosed with prostate cancer this year, and it is the fourth most common cancer worldwide. Fortunately, the vast majority of patients will live for years after being diagnosed and are more likely to die of causes unrelated to a prostate tumor.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
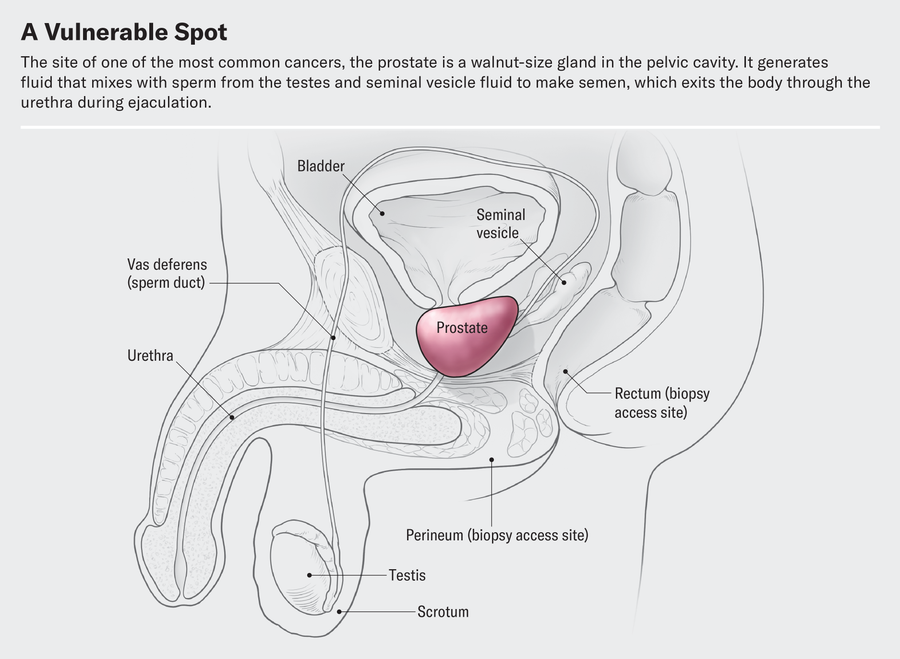
At its most basic level, prostate cancer is a malignancy that occurs in the prostate gland, which produces fluid that mixes with sperm from the testicles to make semen. The prostate is located in front of the rectum, below the bladder and above the penis, and cancer in the gland has four major stages.
Early on, localized tumors show no evidence of extension beyond the prostate gland. A second, “regionally advanced” form of the disease remains close to the prostate. Then there are metastatic prostate cancers, which spread outside the gland to other parts of the body. Treatment of tumors in this category has benefited from improved diagnostic imaging tests. In fact, with these tests, cancer specialists have characterized the fourth category, oligometastatic prostate cancer, a disease stage on a continuum between localized prostate cancer and more broadly dispersed metastatic disease. Major discoveries in the past 10 years have transformed the way we approach each type of prostate cancer, and these advances are likely to continue for decades to come.
The first treatment steps for people with localized cancer involve risk stratification. Through this process, a physician gauges the likelihood of a cancer’s being eliminated or cured by local treatment (usually surgery or radiation) and, if it does abate, of its returning. A physician determines the risk based on PSA results, physical examination of the prostate gland and inspection of cells from the biopsied tumor.
The right course of action for a patient with elevated PSA levels continues to undergo constant revision. Until five to seven years ago, a physician evaluated a person with high PSA by feeling their prostate gland for potentially cancerous abnormalities. Invariably, the next step would be a needle biopsy—an uncomfortable procedure in which the physician obtains snippets of prostate tissue through the rectum.
But we now have a way to biopsy through the perineum—the area between the back of the scrotum and the anal-rectal area. Thanks to technical improvements, it can be done in an outpatient setting without general anesthesia or sedation. The technique reduces the patient’s risk of infection and need for antibiotics because it doesn’t disrupt the bacterial flora in the rectum. In a recent study, researchers compared outcomes in patients who underwent a transrectal biopsy and received antibiotics with those for people who had a transperineal biopsy with minimal to no antibiotics. They found the two approaches comparable in terms of complications from infections.
Even more exciting is the prospect of eliminating biopsies altogether. When a patient has an abnormal PSA value but their rectal examination shows no obvious evidence of cancerous deposits, physicians can now use magnetic resonance imaging (MRI) to look at the prostate and surrounding tissue. MRI scans are best for identifying clinically significant cancers—those that, if left untreated or undiagnosed, could eventually spread. MRI can also uncover more extensive cancer spread or tumors in unusual locations such as the front of the prostate.
David Cheney
Another benefit of MRI procedures is that they identify fewer clinically insignificant cancers—those that are unlikely to cause problems and might best be left alone. In this case, failure to detect certain cancers is a good thing because it spares people unnecessary treatment. In some medical centers in the U.S. and many in Europe, a physician will perform a biopsy only if the MRI scan does reveal evidence of clinical significance. Studies that have compared the two diagnostic approaches—routine biopsy for all patients with elevated PSA levels versus biopsies based on abnormal MRI findings—found they are similarly effective at detecting clinically significant cancers.
Once a patient is diagnosed with prostate cancer, what happens next? For decades the debate over treatment has been just as contentious as the debate over diagnosis. Fortunately, new research from the U.K. has provided some clarity. Investigators there studied several thousand people with elevated PSA levels whose prostate biopsies showed cancer. These patients were randomized to receive surgical removal of the cancerous gland, radiation treatments or no active treatment at all. At the end of 15 years of comprehensive follow-up, about 3 percent of patients in each group had died of prostate cancer, and nearly 20 percent in each group had died of unrelated causes.
Based on the results of this study and others, more people are now being offered “active surveillance” after a prostate cancer diagnosis, in which treatment is either delayed or avoided altogether. Careful monitoring of patients who have not undergone surgery or radiation is becoming more common; it is now being extended even to those with more worrisome tumors. The monitoring involves a range of measures: PSA testing every three to six months, physical examination of the prostate gland and assessment of the patient’s urinary symptoms. Those tests are followed by repeat biopsies at increasing intervals, as long as there are no significant pathological changes.
If a cancer is identified as having either intermediate- or high-risk features, doctors need to track its progression, usually with bone scans using radiopharmaceuticals and with abdominal-pelvic computed tomography (CT) scans, which may show any spread in the areas to which prostate cancer most often metastasizes. Unfortunately, these techniques are not sensitive enough to reliably detect cancer in structures less than a centimeter in diameter, such as lymph nodes. Consequently, small areas of metastatic disease may go undetected. These cases are said to be “understaged.”
Understaging can now be studied through more precise diagnostic testing. Typically patients whose disease is understaged are not treated until the cancer becomes detectable through symptoms such as urination problems or pain. The disease then may require intensive therapies, and there is less of a chance of long-term remission. One technology that can help address understaging is advanced scanning that combines radiodiagnostic positron-emission tomography (PET) with CT.
These scans can detect molecules commonly found in prostate cancer cells, such as prostate-specific membrane antigen (PSMA). If PSMA is present outside the prostate gland, such as in pelvic lymph nodes, the affected areas can be identified, and a plan can be made for targeted radiation treatments or surgical removal.
Let’s consider how PET-CT scanning can be used in clinical practice. One of my patients, a 68-year-old man, was diagnosed with prostate cancer that was localized but had high-risk features. The traditional diagnostic bone and CT scans did not show any evidence of cancer spread outside the prostate. A PET-CT scan for PSMA, however, did reveal the presence of several small deposits of cancer cells in well-defined areas of the pelvis, indicating the cancer had spread to the lymph nodes. This finding prompted treatment that included radiation therapy in the prostate gland and the cancerous lymph nodes, as well as androgen-deprivation therapy (ADT), a treatment that reduces levels of testosterone, the hormone that enables prostate cancer to grow and progress.
The more precise identification of small tumor deposits in a limited number of pelvic lymph nodes—diagnosed as oligometastatic prostate cancer—enabled a new use for an old technology in oncology called metastasis-directed therapy (MDT), which targets cancer-containing lymph nodes or bony areas with radiation. At times, surgical removal of the abnormal lymph nodes may also be incorporated into MDT. Recently published studies on the use of MDT in conjunction with conventional treatments show, in some cases, long-term remission lasting through years of follow-up. Until recently, such a scenario was unthinkable for people whose prostate cancer had spread to their lymph nodes. My patient had the PSMA scan and MDT, as well as a relatively short course of ADT. He is cancer-free for now.
Precise identification of small metastatic deposits has other positive benefits. ADT has for decades been the mainstay for treating many forms of prostate cancer. Patients must continue the therapy for years, sometimes for the rest of their lives. Side effects of ADT are similar to those experienced during menopause. In fact, “andropause” is the term that captures the effects of ADT. Lower levels of testosterone are accompanied by a multitude of symptoms, including but not limited to loss of libido, erectile dysfunction, weight gain, hot flashes, bone loss, cognitive impairment, mood changes, diminished energy, and worsening of preexisting heart and vascular problems.
Studies of MDT for oligometastatic prostate cancer have raised the question of whether ADT could be delayed, administered for a shorter duration or even omitted in patients who otherwise would have required it. By strategically deploying traditional forms of localized treatment—usually surgery to remove the prostate gland or radiation—with added MDT for oligometastatic disease, doctors can significantly shorten the duration of ADT or potentially eliminate it. Such an approach would have been difficult to imagine five years ago. Longer-term follow-up studies will help scientists determine whether some people diagnosed in this fashion can go into an extended remission.
For advanced forms of prostate cancer that have spread to other parts of the body, ADT has been the main treatment. Physicians historically have generally recommended surgical removal of the testicles—the primary source of testosterone—or the administration of other hormones that block the production and action of testosterone. In the mid-1980s I was involved with research on drugs called luteinizing hormone–releasing hormone analogues that lowered testosterone by shutting off the signal in the brain that instructs the testicles to make testosterone. Today newer agents have been added that further lower and block testosterone’s action.
The goal of prostate cancer treatment at later stages is to eliminate multiple sources of testosterone. As noted earlier, testosterone in the body comes predominantly from the testicles; the adrenal glands also produce a small amount. But prostate cancer cells can evolve to produce their own androgens. Testosterone and its active form, dihydrotestosterone (DHT), traverse the membranes of prostate cancer cells and interact with androgen receptors in the cytoplasm, a cell’s liquid interior. The receptors then transport DHT to the nucleus, where it instructs the cancer cell to grow, replicate and spread.
Traditional ADT does little to affect either the production of testosterone by the adrenal glands or androgen-producing prostate cancer cells, and it doesn’t block the activity of androgen receptors. But new approaches to ADT may address these shortcomings. Drug combinations that affect all these processes have substantially improved survival in people with metastatic prostate cancer—and, more important, patients are able to tolerate these more intensive treatment programs.
Instead of just one drug to decrease testosterone, new standards for treatment prescribe combinations of two or even three drugs. In addition to traditional ADT, there are medications such as docetaxel, a chemotherapy, and other new drugs that can block the production of testosterone by the adrenal glands or cancer cells or stop it by interfering with the activity of androgen receptors. All these drug combinations have resulted in meaningful improvements in survival.
Yet another therapy for advanced disease involves the identification of PSMA-expressing cancer cells that can be targeted with pharmaceuticals designed to deliver radioactive bombs. An injectable radiopharmaceutical can be delivered selectively to these cells, leaving healthy cells mostly unaffected. This therapy, lutetium-177-PSMA-617 (marketed as Pluvicto), has been approved by the U.S. Food and Drug Administration for the treatment of prostate cancer that has become resistant to other forms of ADT and chemotherapy. It is likely to become an important therapy for even earlier stages of prostate cancer.
Genetics and genomic testing of patients and cancers have also helped in the quest for improvement of symptoms and longer survival. Some genetic mutations that are known to increase the risk of breast and ovarian cancer have also been associated with a heightened risk of prostate cancer. Testing for such mutations is becoming much more common, and patients who have them can be treated with specific therapies that block their deleterious effects, leading to better outcomes.
An understanding of the type of mutation is also critical—for both patients and their family members. Germline mutations are inherited from a patient’s biological parents by every cell in the body. These mutations can be passed along to the patient’s children. A somatic mutation, in contrast, is not inherited but develops in the cancer itself. Targeted therapies designed specifically to correct the effects of either germline or somatic mutations have produced significant improvements in patient longevity. Some of the most commonly recognized cancer mutations—either somatic or germline—are those in BRCA genes, which have been associated with early-onset breast and ovarian cancer.
When researchers studied cancer in families with BRCA mutations, they uncovered many cases of prostate cancer. This finding led to the discovery that BRCA mutations appeared in both men and women in these families. The mutations change the way DNA is repaired, introducing defects that can result in cancer formation. Drugs have now been developed that treat cancers linked to the BRCA mutations. Several such drugs—those in a class called poly(ADP-ribose) polymerase (PARP) inhibitors—have recently received FDA approval for use as a treatment in people with these mutations. This research has led to more widespread genetic testing of patients with prostate cancer and, when germline mutations are found, family genetic counseling.
All these advances have occurred over the past decade—an incredibly short interval in the context of cancer oncology. Current options for early-stage prostate cancer enable physicians and patients to feel more at ease with conservative choices rather than immediate interventions with negative side effects. For patients whose cancers are advanced at initial diagnosis or progress and become metastatic, the treatment of oligometastases now often leads to long-term remission and requires fewer treatments with harmful systemic side effects. For those with more widespread metastatic disease, their cancer can now be managed with improved therapeutics based on a better understanding of disease biology. These new strategies have begun to transform this once rapidly fatal disease into a chronic condition that people can live with for years or even for their full life expectancy.