Aromaticity’s dark alter-ego is ready to emerge into the sunlight. James Mitchell Crow talks to the scientists trying to exploit the instability
Discovered at the dawn of the modern chemical age, aromaticity is a concept deeply familiar to multiple generations of chemists. Benzene was first isolated in 1825 by Michael Faraday, from an oily residue of the compressed gas used to light the lamps of his Royal Institution laboratory, and its stable and unreactive nature relative to its peers was soon recognised. Our understanding of benzene’s bonding, structure and stability has matured in step with advances in chemical theory. Over many decades, the reliable stabilising influence of the benzene ring has been usefully incorporated into countless molecular structures.
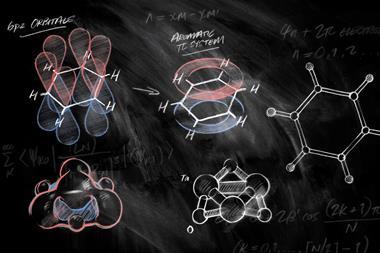
But put benzene under the spotlight and less familiar behaviour emerges. As benzene becomes photoexcited its character shifts radically. The molecule synonymous with stability becomes highly unstable, rearranging near-instantaneously to form fulvene and benzvalene, an explosive white powder. In the photoexcited state, benzene is antiaromatic.
‘From a chemical point of view, aromatic molecules are boring,’ says Igor Alabugin from Florida State University, US. ‘They are too stable, they’re happy with themselves,’ he says. But antiaromatic molecules – which will do whatever they can to escape this unstable state – are anything but dull, Alabugin adds. ‘Antiaromatic molecules are anxious, reactive, always ready to surprise you.’
As antiaromatic behaviour is increasingly understood, potential uses for its extreme reactivity are beginning to emerge. A century or more after chemists first capitalised on aromaticity for molecular assembly, real world potential applications for antiaromaticity – not least by harnessing the ability to tip aromatic molecules into an antiaromatic state at the flick of a light switch – are now being explored. The possible applications of antiaromaticity are diverse, from medicine to clean energy.
Pseudo science
When chemists first described the key structural features of benzene and related aromatic compounds – most notably, the planar cyclic π-conjugated structure, which its π-electrons could race around in a delocalised ring – one key point was missed. ‘People started to generalise and to think that all π-conjugated cyclic systems must behave similarly,’ says Judy Wu, a theoretical chemist from University of Houston in Texas, US. ‘So the first shock was when cyclooctatetraene [COT] was prepared.’
The expectation was that COT would behave like an aromatic compound, but it doesn’t. COT – a conjugated eight-membered cyclic system with one extra π-bond than benzene – doesn’t show enhanced stability. Initially, such molecules – which looked cyclic and π-conjugated, but did not display aromatic properties – were dubbed pseudo-aromatic, says Wu. ‘It was this wishy-washy term for π-conjugated systems that we don’t know where to place.’
COT’s surprising properties paled compared to that of cyclobutadiene, a four-membered ring with one fewer conjugated π-bond than benzene, notes Alabugin. Cyclobutadiene is not actually difficult to make. ‘If you take two molecules of acetylene, and dimerise it, you make cyclobutadiene,’ he says. Acetylene is relatively stable, and the dimerisation reaction is energetically favourable – but the moment that cyclobutene is formed, it’s gone. ‘What you make is something unusual and unstable,’ Alabugin says. ‘Cyclobutadiene doesn’t persist at all.’
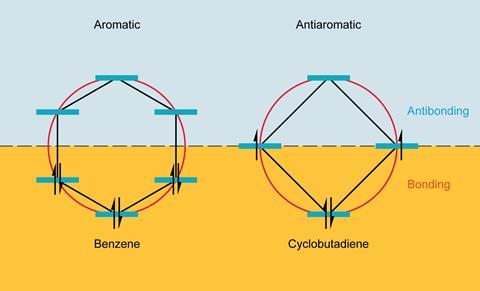
The term antiaromaticity was introduced by US chemist Ronald Breslow in 1967 to describe the characteristic destabilisation of such molecules. The difference between aromatic and antiaromatic compounds comes down to the number of π-electrons in their cyclic conjugated system. As German physicist Erich Hückel had formalised, planar cyclic structures with 4n + 2 π-electrons are aromatic, their molecular orbitals arrange such that all their π-electrons occupy bonding orbitals. Rings with 4n π-electrons – such as COT and cyclobutadiene – are antiaromatic, with two of their π-electrons forced to occupy a non- or antibonding orbital, a very unstable state.
Electron counting suggests COT should be antiaromatic, but it escapes this fate by puckering up out of the plane, so that its π-orbitals no longer overlap and it becomes non-aromatic instead. Cyclobutadiene, too small to repeat the trick, sheds its antiaromaticity by instantly dimerising.
Aromaticity is all great, but anti-aromaticity is the real fun
After a burst of antiaromaticity research activity in the 1960s and 70s, interest in the concept faded, simply because it didn’t seem terribly useful. ‘If you had asked me about antiaromaticity six years ago, I would have said I wasn’t interested,’ says Wu. ‘You can’t capture it, so what’s the point? Chemists spent some 60 years just trying to trap cyclobutadiene, the simplest antiaromatic molecule.’
Some antiaromatics do remain difficult to capture. ‘Cyclobutadiene is still elusive,’ Alabugin says. ‘Only about 10 years ago, there was an article in Science claiming to have made cyclobutadiene – when, in fact, they didn’t.’
But recently, some antiaromatic molecules have been tamed. One of the pioneers is Mike Haley at the University of Oregon, US – who turned Wu on to the concept. ‘In 2018, I met with Mike Haley to discuss some chemistry related to aromaticity,’ Wu says. ‘And he said “You know, aromaticity is all great, but anti-aromaticity is the real fun”.’
On the brink
When Haley got into antiaromaticity research around 15 years ago, few other groups were interested in the idea, he says. ‘After the 1970s, making weird hydrocarbon molecules just because they were antiaromatic went out of fashion,’ Haley says.
Initially, antiaromaticity research was a side project in the Haley lab. The work was prompted by a student looking for a project a little different to the research topics the group was currently working on. ‘The student said to me “Do you have any crazy ideas in the back of your mind?”’ Haley recalls. ‘And of course, professors always have crazy ideas in the backs of their minds.’
At the time, in 2009, interest had been growing in organic molecules with conductive or semiconductive properties. Haley suspected molecules with antiaromatic character would fit the bill. With two π-electrons parked in an antibonding orbital, antiaromatics have a high energy highest occupied molecular orbital (Homo), and not much of an energy jump to the lowest unoccupied molecular orbital (Lumo). ‘The small Homo–Lumo gap, one of the hallmarks of antiaromatic molecules, means they might be useful for electronic applications,’ Haley says.
We could clamp some aromatic rings to an antiaromatic core to make molecules that were at least stable enough to isolate
Haley’s idea was to take pentacene – an aromatic linear string of five fused benzene rings, which had p-type organic semiconductor properties – and swap two of the rings in the string with five-membered rings, to form a linear pentacycle with an indacene core. ‘The big difference to pentacene is that, by taking two carbon atoms away, it’s now a 20 π-electron system, which makes it antiaromatic,’ Haley says.
The first challenge Haley and his student faced was to make these molecules, given the inherent instability of their target. At the time, being able to make such systems in sufficient quantity to explore their properties was a real barrier, Wu says. ‘People had realised that we could clamp some aromatic rings to an antiaromatic core to make molecules that were at least stable enough to isolate – but the yields were low,’ says Wu.
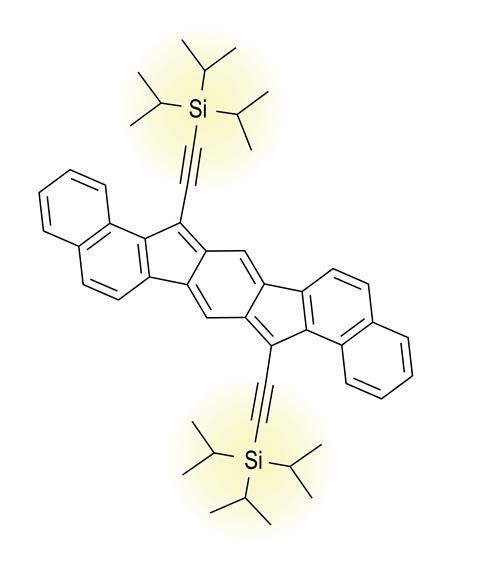
For Haley’s indacene-type antiaromatics, the key step forward was to attach a bulky (trialkylsilyl)acetylene (TIPS) side group to each of the molecule’s two five-membered rings. ‘TIPS acetylene is like a giant umbrella-shaped protecting group that stabilises the antiaromatic core, and that’s what allowed us to be able to isolate these molecules,’ Haley says. ‘Anything smaller than TIPS acetylene, then the molecule doesn’t survive.’
In 2009, groups in Japan and the US independently published metal-catalysed methods for making pentalene – an 8π-antiaromatic structure consisting of two fused five-membered rings – attached to stabilising benzene rings. ‘It was only after these groups found ways of making π-expanded 4n cores with higher yields that we could finally see if we could do something interesting with them,’ Wu says.
For Haley’s aromatic-fused, TIPS-protected indacene-based molecules, the enhanced conductivity imparted by the narrow Homo–Lumo energy gap is only one aspect of its interesting electronic properties. ‘These molecules want to escape the destabilising feature of antiaromaticity, so they readily accept electrons – like pentacene – but more unusually they also readily give up electrons,’ Haley says. Accepting or donating electrons allows the molecule to escape its 4nπ-electron status – and enables the molecule to act either as a p-type or an n-type semiconductor. ‘We’ve made molecules that behave as the active layer in an organic field effect transistor, so we now know this idea works,’ Haley says.
Over the years, Haley estimates the team has made close to 100 variants of the structure, exploring how altering the molecular core and periphery alters its electronic properties. Most recently, the team has explored what happens on going from one benzene ring in the centre of the structure, to two or even three.
‘If you have an antiaromatic molecule, one of the reasons they’re so destabilised is that you’ve got two electrons in the π* anti-bonding orbital,’ he says. Adding rings to the indacene’s antiaromatic core starts to draw these electrons apart, he says. ‘By increasing the space, the two electrons are no longer closely correlated, and the molecule now behaves like a diradical.’ The two electrons in these molecules can be flipped between the spin paired ‘singlet’ state and spin parallel ‘triplet’ states at much closer to room temperature that typical diradical species, which suggests potential for spin-based computing or memory, Haley says.
‘Are we ever going to get there? I don’t know – but I think that our work figuring out how to rationally tune the singlet–triplet and Homo–Lumo energy gaps allows you to begin to understand how to tailor the molecule for these applications,’ Haley says. ‘We’ve shown that we can tune the singlet triplet gap anywhere from 3.2–9.6kCal [13–40kJ], which is a huge window.’
Jekyll and Hyde
‘Mike Haley’s molecules are interesting because they balance on the brink between stability and being interesting electronically,’ Alabugin says. ‘They are not too unstable, but close enough to have this tuneable electronic nature.’
Rather than balance antiaromaticity’s instability, an alternative approach is to exploit it, as a way to make molecules that are inaccessible by other routes. Using light to access the antiaromatic state is proving to be a powerful tool, Alabugin adds. ‘Photochemistry is this unique way of converting aromatic into antiaromatic, and that allows you to get some new chemistry,’ he says.
For benzene, the light-driven change in character is so dramatic that it is akin to a molecular Dr Jekyll and Mr Hyde, according to Henrik Ottosson from Uppsala University in Sweden: its familiar stable aromatic character tipping into a violent Mr Hyde when photoexcited.
The concept that aromatic molecules become antiaromatic when photoexcited was first predicted by perturbation molecular orbital theory in 1972, by Colin Baird from the University of Western Ontario, Canada, says Ottosson. Baird even predicted the impact that this destabilisation would have on a molecule like benzene, he adds. ‘Baird wrote in his original paper “The antiaromaticity associated with the triplet state of benzenoid hydrocarbons is predicted to yield drastic changes in the intermolecular reactivity compared to the ground state,”’ Ottosson says. Today, the fact that many 4n + 2 π-electron species are antiaromatic in the lowest π-π* excited states is known as Baird’s rule – but for four decades after Baird’s work was published, the paper attracted very little attention.
Ottosson started researching the subject in the early 2000s, when investigating some photochemical reactions that could be explained in terms of aromaticity effects. ‘It was very much a side project until 2010, when a collaborator in Copenhagen, Kristine Kilså, and I started to see more papers appearing on excited state aromaticity, and we thought we should write a brief review paper together.’
As the team dug into the topic, they found all the theory already there in the literature, adding to Baird’s contribution. ‘But then we realised how broadly we could apply the theory to explain different organic photoreactions and other excited state behaviour,’ Ottosson says. The ‘brief review’, when finally published in 2014, stretched to almost 50 pages.
Ottosson’s experience is echoed by that of Wu. The more we’ve got to grips with antiaromaticity, she says, the more we see the role it has been playing all along in well-established chemistry– from explaining photoexcited state reactivity to biological processes.
‘I was looking at some photochemical proton transfer reactions – when I realised that they are examples of antiaromaticity relief,’ Wu says. Wu had noticed that many aromatic compounds undergo proton transfer when photoexcited. ‘In that state, the aromatic compound becomes antiaromatic, and losing a proton is the easiest way – near barrierless in many cases – to lose that antiaromaticity,’ says Wu. ‘We realised that nearly all the important chemical and biological examples where you find excited state proton transfer have this anti-aromaticity relief involved.’ The process is even key to the way that DNA molecules minimise photodegradation when exposed to UV light.
Reaction control
One potential application for using light to convert a stable aromatic compound into a reactive antiaromatic could be to break molecules down at their end of life. Light treatment might offer a way to decompose aromatic drug molecules in wastewater, preventing these bioactive compounds from entering the environment, Ottosson says.
The potent reactivity of antiaromatic molecules is also being applied to new reaction design. ‘In my group, we have two examples where we have used antiaromaticity to accomplish something that was difficult or impossible before,’ Alabugin says.
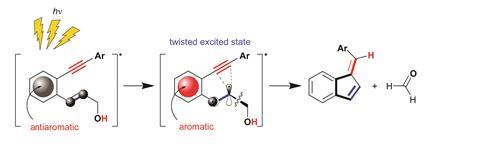
The first example arose from Alabugin’s long-standing interest in cycloaromatisation reactions, including the Bergman cyclisation. ‘This is an interesting reaction because it converts a molecule with two triple bonds into a diradical, which is unstable - but you simultaneously make a benzene ring, and so aromaticity pays for making something unstable,’ Alabugin says. There are four potential ways that such diradical cycloaromatisations might proceed, and four different cyclised products that would result. One of these four cycloaromatisations was unknown. ‘So we designed a way to do it, using systems incorporating a benzene ring.’
In a light-driven reaction, the molecule becomes antiaromatic, then twists to escape the antiaromaticity – which favours the unusual cyclisation. ‘By inducing transient antiaromaticity, we achieved this missing cycloaromatisation reaction,’ Alabugin says.
As well as a potentially useful synthetic transformation, the reaction has other possible applications, Alabugin adds. ‘We designed this reaction in a way that, simultaneously with making the cycle, it releases a molecule of formaldehyde, or other aldehyde or ketone,’ he says. Formaldehyde usually exists as a complex polymeric mixture, so the ability to produce molecular formaldehyde on demand could be useful, Alabugin says. The team has also used the reaction as a photo-triggered way to release other ketones or aldehydes, which suggests it could have applications for controlled drug release.
In the team’s second example, they accessed antiaromaticity by converting a polycyclic aromatic into a dianion in two steps. ‘When we add the second negative charge by reducing one of the benzene rings, it adds as far as possible from the first negative charge,’ Alabugin says. ‘The molecule became anti-aromatic, but in a very localised way, directed by the location of the pre-existing negative charge.’ The molecule escapes antiaromaticity at that hotspot by undergoing cyclisation, producing a molecule that is like a piece of graphene 10 rings in size – but where one of the cycles is a five-membered ring. ‘By localising the antiaromaticity we could embed a five membered cycle into graphene, which would be something that’s otherwise difficult to do,’ Alabugin says.
Illuminating
Where aromatic molecules become antiaromatic in the excited state, molecules with antiaromatic character in the ground state become aromatic, gaining potentially useful stabilisation. One line of research that looks to exploit this fact is in the development of materials for high efficiency solar panels.
Organic molecules that undergo a process called singlet fission can absorb a single high energy photon to enter the singlet excited state, then undergo a fission process to generate two excitons of lower energy in the triplet state. ‘So you get two for the price of one,’ Ottosson says. If such a material were to be coated onto a silicon solar cell, it would boost the theoretical maximum efficiency of the device up to 44%.
I think one shouldn’t be afraid of antiaromaticity
Molecules that have formal antiaromatic character in the ground state – but remain stable because they escape antiaromaticity’s effects by, for example, bending out of plane like cyclooctatetraene does – could be ideal starting points for developing singlet fission materials, Ottosson says.
‘We may design chromophore molecules for singlet fission photovoltaics with increased photostability, using the excited state aromaticity concept,’ Ottosson says. A photostable molecule might enhance the odds of successful singlet fission and twin exciton generation, as well as displaying improved longevity and working lifetime up on a rooftop.
Excited state aromatic and antiaromatic theory also offers a rationale for designing chromophores with just the right energy levels to deliver triplet excitons to silicon with sufficient energy to clear silicon’s bandgap, thereby enabling the captured sunlight to be used. ‘With singlet fission, the energy levels have to be just right,’ Haley says. ‘Some of the molecules that we’ve made have been calculated to be potentially useful for singlet fission. It’s an obvious natural extension of our research, and we’re working on it,’ he adds.
‘Being able to understand antiaromaticity allows you to open new doors, and I hope that we will see more of it in the future,’ Alabugin says. ‘I think one shouldn’t be afraid of antiaromaticity. If something is unstable, that means it’s ready to be converted into something else you might just need.’
James Mitchell Crow is a science writer based in Melbourne, Australia


















2 readers' comments