Abstract
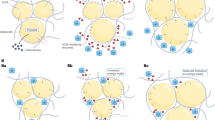
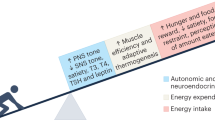
One of the biggest challenges in the management of obesity is the prevention of weight regain after successful weight loss. Weight regain after weight loss has large interindividual variation. Although many factors probably contribute to this variation, we hypothesize that variability in biological responses associated with weight loss-induced shrinking of subcutaneous adipocytes has an important role. In this Review, we show that weight loss-induced variations in cellular stress, extracellular matrix remodelling, inflammatory responses, adipokine secretion and lipolysis seem to be associated with the amount of weight that is regained after successful weight loss. Weight regain could therefore, at least in part, depend on a combination of these factors. Further research on the causality of these associations could aid the development of effective strategies to prevent weight regain after successful weight loss.
Key points
-
Weight regain after successful weight loss is a major problem for many individuals, and many factors are probably involved in driving weight regain.
-
Loss of fat mass induces shrinkage of adipocytes, which is accompanied by cell stress, inflammation, altered adipokine secretion and reduced lipolysis.
-
In the absence of extracellular matrix remodelling during adipocyte shrinkage, mechanical stress builds up between the cell and the oversized extracellular matrix, which inhibits lipolysis and the release of fatty acids from adipocytes.
-
Weight loss induces an inflammatory response in adipose tissue.
-
Evidence for the involvement of epigenetic modifications, in particular microRNAs, in weight regain is sparse.
-
Knowledge of weight regain after weight loss is mainly based on associations, and so research into the causality of such associated factors is needed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 387, 1377–1396 (2016).
Santos, I., Sniehotta, F. F., Marques, M. M., Carraca, E. V. & Teixeira, P. J. Prevalence of personal weight control attempts in adults: a systematic review and meta-analysis. Obes. Rev. 18, 32–50 (2017).
Anderson, J. W., Konz, E. C., Frederich, R. C. & Wood, C. L. Long-term weight-loss maintenance: a meta-analysis of US studies. Am. J. Clin. Nutr. 74, 579–584 (2001).
Look AHEAD Research Group. Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring) 22, 5–13 (2014).
Christou, N. V., Look, D. & Maclean, L. D. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann. Surg. 244, 734–740 (2006).
Magro, D. O. et al. Long-term weight regain after gastric bypass: a 5-year prospective study. Obes. Surg. 18, 648–651 (2008).
Odom, J. et al. Behavioral predictors of weight regain after bariatric surgery. Obes. Surg. 20, 349–356 (2010).
Schwartz, M. W. et al. Obesity pathogenesis: an Endocrine Society scientific statement. Endocr. Rev. 38, 267–296 (2017).
Hopkins, M. & Blundell, J. E. Energy balance, body composition, sedentariness and appetite regulation: pathways to obesity. Clin. Sci. 130, 1615–1628 (2016).
MacLean, P. S., Blundell, J. E., Mennella, J. A. & Batterham, R. L. Biological control of appetite: a daunting complexity. Obesity (Silver Spring) 25 (Suppl. 1), S8–S16 (2017).
Dulloo, A. G., Jacquet, J., Miles-Chan, J. L. & Schutz, Y. Passive and active roles of fat-free mass in the control of energy intake and body composition regulation. Eur. J. Clin. Nutr. 71, 353–357 (2017).
Melby, C. L., Paris, H. L., Foright, R. M. & Peth, J. Attenuating the biologic drive for weight regain following weight loss: must what goes down always go back up? Nutrients 9, 468 (2017).
Ochner, C. N., Barrios, D. M., Lee, C. D. & Pi-Sunyer, F. X. Biological mechanisms that promote weight regain following weight loss in obese humans. Physiol. Behav. 120, 106–113 (2013).
Sumithran, P. & Proietto, J. The defence of body weight: a physiological basis for weight regain after weight loss. Clin. Sci. 124, 231–241 (2013).
Mariman, E. C. Human biology of weight maintenance after weight loss. J. Nutrigenet. Nutrigenomics 5, 13–25 (2012).
Sumithran, P. et al. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 365, 1597–1604 (2011).
Munzberg, H., Laque, A., Yu, S., Rezai-Zadeh, K. & Berthoud, H. R. Appetite and body weight regulation after bariatric surgery. Obes. Rev. 16 (Suppl. 1), 77–90 (2015).
Lean, M. E. & Malkova, D. Altered gut and adipose tissue hormones in overweight and obese individuals: cause or consequence? Int. J. Obes. (Lond.) 40, 622–632 (2016).
Verhoef, S. P., Camps, S. G., Bouwman, F. G., Mariman, E. C. & Westerterp, K. R. Physiological response of adipocytes to weight loss and maintenance. PLOS ONE 8, e58011 (2013).
Haczeyni, F., Bell-Anderson, K. S. & Farrell, G. C. Causes and mechanisms of adipocyte enlargement and adipose expansion. Obes. Rev. 19, 406–420 (2018).
Park, K. W., Halperin, D. S. & Tontonoz, P. Before they were fat: adipocyte progenitors. Cell Metab. 8, 454–457 (2008).
Engin, A. Fat cell and fatty acid turnover in obesity. Adv. Exp. Med. Biol. 960, 135–160 (2017).
Spalding, K. L. et al. Dynamics of fat cell turnover in humans. Nature 453, 783–787 (2008).
Tchoukalova, Y. D. et al. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc. Natl Acad. Sci. USA 107, 18226–18231 (2010).
Arner, E. et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes 59, 105–109 (2010).
Jonker, J. W. et al. A PPARgamma-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature 485, 391–394 (2012).
Arner, P., Andersson, D. P., Backdahl, J., Dahlman, I. & Ryden, M. Weight gain and impaired glucose metabolism in women are predicted by inefficient subcutaneous fat cell lipolysis. Cell Metab. 28, 45–54 (2018).
Khan, T. et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol. 29, 1575–1591 (2009).
Henegar, C. et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 9, R14 (2008).
Muir, L. A. et al. Adipose tissue fibrosis, hypertrophy, and hyperplasia: correlations with diabetes in human obesity. Obesity (Silver Spring) 24, 597–605 (2016).
Divoux, A. et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 59, 2817–2825 (2010).
Trayhurn, P. Hypoxia and adipocyte physiology: implications for adipose tissue dysfunction in obesity. Annu. Rev. Nutr. 34, 207–236 (2014).
Sun, K., Tordjman, J., Clement, K. & Scherer, P. E. Fibrosis and adipose tissue dysfunction. Cell Metab. 18, 470–477 (2013).
Lin, N. & Simon, M. C. Hypoxia-inducible factors: key regulators of myeloid cells during inflammation. J. Clin. Invest. 126, 3661–3671 (2016).
Lin, Q. & Yun, Z. The hypoxia-inducible factor pathway in adipocytes: the role of HIF-2 in adipose inflammation and hypertrophic cardiomyopathy. Front. Endocrinol. (Lausanne) 6, 39 (2015).
Pasarica, M. et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58, 718–725 (2009).
Goossens, G. H. et al. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation 124, 67–76 (2011).
Vink, R. G. et al. Diet-induced weight loss decreases adipose tissue oxygen tension with parallel changes in adipose tissue phenotype and insulin sensitivity in overweight humans. Int. J. Obes. (Lond.) 41, 722–728 (2017).
Ellulu, M. S., Patimah, I., Khaza’ai, H., Rahmat, A. & Abed, Y. Obesity and inflammation: the linking mechanism and the complications. Arch. Med. Sci. 13, 851–863 (2017).
Domenis, R. et al. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci. Rep. 8, 13325 (2018).
Engin, A. B. Adipocyte-macrophage cross-talk in obesity. Adv. Exp. Med. Biol. 960, 327–343 (2017).
Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 115, 911–919 (2005).
Rosen, E. D. & Spiegelman, B. M. What we talk about when we talk about fat. Cell 156, 20–44 (2014).
Kratz, M. et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 20, 614–625 (2014).
Alligier, M. et al. Subcutaneous adipose tissue remodeling during the initial phase of weight gain induced by overfeeding in humans. J. Clin. Endocrinol. Metab. 97, E183–E192 (2012).
Shimobayashi, M. et al. Insulin resistance causes inflammation in adipose tissue. J. Clin. Invest. 128, 1538–1550 (2018).
Maclean, P. S., Bergouignan, A., Cornier, M. A. & Jackman, M. R. Biology’s response to dieting: the impetus for weight regain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R581–R600 (2011).
Vink, R. G., Roumans, N. J., Arkenbosch, L. A., Mariman, E. C. & van Baak, M. A. The effect of rate of weight loss on long-term weight regain in adults with overweight and obesity. Obesity (Silver Spring) 24, 321–327 (2016).
Lenz, M. et al. Estimating real cell size distribution from cross-section microscopy imaging. Bioinformatics 32, i396–i404 (2016).
Isakson, P., Hammarstedt, A., Gustafson, B. & Smith, U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes 58, 1550–1557 (2009).
Rossmeislova, L. et al. Weight loss improves the adipogenic capacity of human preadipocytes and modulates their secretory profile. Diabetes 62, 1990–1995 (2013).
Vink, R. G., Roumans, N. J., Mariman, E. C. & van Baak, M. A. Dietary weight loss-induced changes in RBP4, FFA, and ACE predict weight regain in people with overweight and obesity. Physiol. Rep. 5, e13450 (2017).
Rosenbaum, M., Kissileff, H. R., Mayer, L. E., Hirsch, J. & Leibel, R. L. Energy intake in weight-reduced humans. Brain Res. 1350, 95–102 (2010).
Bouwman, F., Renes, J. & Mariman, E. A combination of protein profiling and isotopomer analysis using matrix-assisted laser desorption/ionization-time of flight mass spectrometry reveals an active metabolism of the extracellular matrix of 3T3-L1 adipocytes. Proteomics 4, 3855–3863 (2004).
Wang, P. et al. Insulin modulates the secretion of proteins from mature 3T3-L1 adipocytes: a role for transcriptional regulation of processing. Diabetologia 49, 2453–2462 (2006).
Mariman, E. C. & Wang, P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell. Mol. Life Sci. 67, 1277–1292 (2010).
Rossmeislova, L., Malisova, L., Kracmerova, J. & Stich, V. Adaptation of human adipose tissue to hypocaloric diet. Int. J. Obes. (Lond.) 37, 640–650 (2013).
Duncan, R. E., Ahmadian, M., Jaworski, K., Sarkadi-Nagy, E. & Sul, H. S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 27, 79–101 (2007).
Liu, Y. et al. Accumulation and changes in composition of collagens in subcutaneous adipose tissue after bariatric surgery. J. Clin. Endocrinol. Metab. 101, 293–304 (2016).
Schwarz, U. S. et al. Calculation of forces at focal adhesions from elastic substrate data: the effect of localized force and the need for regularization. Biophys. J. 83, 1380–1394 (2002).
Mutch, D. M. et al. A distinct adipose tissue gene expression response to caloric restriction predicts 6-mo weight maintenance in obese subjects. Am. J. Clin. Nutr. 94, 1399–1409 (2011).
Roumans, N. J. et al. Weight loss-induced stress in subcutaneous adipose tissue is related to weight regain. Br. J. Nutr. 115, 913–920 (2016).
Schneider, G. B., Hamano, H. & Cooper, L. F. In vivo evaluation of hsp27 as an inhibitor of actin polymerization: hsp27 limits actin stress fiber and focal adhesion formation after heat shock. J. Cell. Physiol. 177, 575–584 (1998).
Roumans, N. J. T. et al. Weight loss-induced cellular stress in subcutaneous adipose tissue and the risk for weight regain in overweight and obese adults. Int. J. Obes. (Lond.) 41, 894–901 (2017).
Roumans, N. J. et al. Variation in extracellular matrix genes is associated with weight regain after weight loss in a sex-specific manner. Genes Nutr. 10, 56 (2015).
Veit, G. et al. Collagen XXIII, novel ligand for integrin α2β1 in the epidermis. J. Biol. Chem. 286, 27804–27813 (2011).
Schluterman, M. K. et al. Loss of fibulin-5 binding to β1 integrins inhibits tumor growth by increasing the level of ROS. Dis. Model. Mech. 3, 333–342 (2010).
Roumans, N. J., Vink, R. G., Fazelzadeh, P., van Baak, M. A. & Mariman, E. C. A role for leukocyte integrins and extracellular matrix remodeling of adipose tissue in the risk of weight regain after weight loss. Am. J. Clin. Nutr. 105, 1054–1062 (2017).
Roumans, N. J. T., Wang, P., Vink, R. G., van Baak, M. A. & Mariman, E. C. M. Combined analysis of stress- and ECM-related genes in their effect on weight regain. Obesity (Silver Spring) 26, 492–498 (2018).
MacLean, P. S., Higgins, J. A., Giles, E. D., Sherk, V. D. & Jackman, M. R. The role for adipose tissue in weight regain after weight loss. Obes. Rev. 16 (Suppl. 1), 45–54 (2015).
Ge, F. et al. Facilitated long chain fatty acid uptake by adipocytes remains upregulated relative to BMI for more than a year after major bariatric surgical weight loss. Obesity (Silver Spring) 24, 113–122 (2016).
Grenier-Larouche, T. et al. Fatty acid metabolic remodeling during type 2 diabetes remission after bariatric surgery. Diabetes 66, 2743–2755 (2017).
Bouwman, F. G., Wang, P., van Baak, M., Saris, W. H. & Mariman, E. C. Increased β-oxidation with improved glucose uptake capacity in adipose tissue from obese after weight loss and maintenance. Obesity (Silver Spring) 22, 819–827 (2014).
Eastman, Q. Very low calorie diet makes adipocytes “scream”. J. Proteome Res. 8, 5408 (2009).
Vink, R. G. et al. Adipose tissue meal-derived fatty acid uptake before and after diet-induced weight loss in adults with overweight and obesity. Obesity (Silver Spring) 25, 1391–1399 (2017).
Santosa, S., Hensrud, D. D., Votruba, S. B. & Jensen, M. D. The influence of sex and obesity phenotype on meal fatty acid metabolism before and after weight loss. Am. J. Clin. Nutr. 88, 1134–1141 (2008).
Johansson, L. E. et al. Differential gene expression in adipose tissue from obese human subjects during weight loss and weight maintenance. Am. J. Clin. Nutr. 96, 196–207 (2012).
Van Pelt, D. W., Guth, L. M., Wang, A. Y. & Horowitz, J. F. Factors regulating subcutaneous adipose tissue storage, fibrosis, and inflammation may underlie low fatty acid mobilization in insulin-sensitive obese adults. Am. J. Physiol. Endocrinol. Metab. 313, E429–E439 (2017).
Schwartz, A. & Doucet, E. Relative changes in resting energy expenditure during weight loss: a systematic review. Obes. Rev. 11, 531–547 (2010).
Camps, S. G., Verhoef, S. P. & Westerterp, K. R. Weight loss, weight maintenance, and adaptive thermogenesis. Am. J. Clin. Nutr. 97, 990–994 (2013).
Hall, K. D. & Kahan, S. Maintenance of lost weight and long-term management of obesity. Med. Clin. North Am. 102, 183–197 (2018).
Camps, S. G. et al. Weight loss-induced changes in adipose tissue proteins associated with fatty acid and glucose metabolism correlate with adaptations in energy expenditure. Nutr. Metab. (Lond.) 12, 37 (2015).
Bouwman, F. G. et al. The physiologic effects of caloric restriction are reflected in the in vivo adipocyte-enriched proteome of overweight/obese subjects. J. Proteome Res. 8, 5532–5540 (2009).
Jokinen, R. et al. Adipose tissue mitochondrial capacity associates with long-term weight loss success. Int. J. Obes. (Lond.) 42, 817–825 (2017).
Marquez-Quinones, A. et al. Adipose tissue transcriptome reflects variations between subjects with continued weight loss and subjects regaining weight 6 mo after caloric restriction independent of energy intake. Am. J. Clin. Nutr. 92, 975–984 (2010).
Chen, Y., Yang, J., Nie, X., Song, Z. & Gu, Y. Effects of bariatric surgery on change of brown adipocyte tissue and energy metabolism in obese mice. Obes. Surg. 28, 820–830 (2018).
Vijgen, G. H. et al. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J. Clin. Endocrinol. Metab. 97, E1229–E1233 (2012).
Dadson, P. et al. Brown adipose tissue lipid metabolism in morbid obesity: effect of bariatric surgery-induced weight loss. Diabetes Obes. Metab. 20, 1280–1288 (2018).
Barquissau, V. et al. Caloric restriction and diet-induced weight loss do not induce browning of human subcutaneous white adipose tissue in women and men with obesity. Cell Rep. 22, 1079–1089 (2018).
Neinast, M. D. et al. Activation of natriuretic peptides and the sympathetic nervous system following Roux-en-Y gastric bypass is associated with gonadal adipose tissues browning. Mol. Metab. 4, 427–436 (2015).
Fabbiano, S. et al. Caloric restriction leads to browning of white adipose tissue through type 2 immune signaling. Cell Metab. 24, 434–446 (2016).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Siiteri, P. K. Adipose tissue as a source of hormones. Am. J. Clin. Nutr. 45, 277–282 (1987).
Wang, P., Mariman, E., Renes, J. & Keijer, J. The secretory function of adipocytes in the physiology of white adipose tissue. J. Cell. Physiol. 216, 3–13 (2008).
Choi, C. H. J. & Cohen, P. Adipose crosstalk with other cell types in health and disease. Exp. Cell Res. 360, 6–11 (2017).
Hocking, S. L., Wu, L. E., Guilhaus, M., Chisholm, D. J. & James, D. E. Intrinsic depot-specific differences in the secretome of adipose tissue, preadipocytes, and adipose tissue-derived microvascular endothelial cells. Diabetes 59, 3008–3016 (2010).
Strohacker, K., McCaffery, J. M., MacLean, P. S. & Wing, R. R. Adaptations of leptin, ghrelin or insulin during weight loss as predictors of weight regain: a review of current literature. Int. J. Obes. (Lond.) 38, 388–396 (2014).
Wang, P. et al. Blood profile of proteins and steroid hormones predicts weight change after weight loss with interactions of dietary protein level and glycemic index. PLOS ONE 6, e16773 (2011).
Wang, P. et al. Circulating ACE is a predictor of weight loss maintenance not only in overweight and obese women, but also in men. Int. J. Obes. (Lond.) 36, 1545–1551 (2012).
Rosenbaum, M. et al. Effects of weight change on plasma leptin concentrations and energy expenditure. J. Clin. Endocrinol. Metab. 82, 3647–3654 (1997).
Tamez, M. et al. Adipocyte size and leptin receptor expression in human subcutaneous adipose tissue after Roux-en-Y gastric bypass. Obes. Surg. 27, 3330–3332 (2017).
Bluher, M. & Mantzoros, C. S. From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century. Metabolism 64, 131–145 (2015).
Qi, Y. et al. Adiponectin acts in the brain to decrease body weight. Nat. Med. 10, 524–529 (2004).
Park, S., Kim, D. S., Kwon, D. Y. & Yang, H. J. Long-term central infusion of adiponectin improves energy and glucose homeostasis by decreasing fat storage and suppressing hepatic gluconeogenesis without changing food intake. J. Neuroendocrinol. 23, 687–698 (2011).
Smith, U. & Kahn, B. B. Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. J. Intern. Med. 280, 465–475 (2016).
Noy, N. Vitamin A in regulation of insulin responsiveness: mini review. Proc. Nutr. Soc. 75, 212–215 (2016).
Brestoff, J. R. & Artis, D. Immune regulation of metabolic homeostasis in health and disease. Cell 161, 146–160 (2015).
Bernstein, K. E. et al. A modern understanding of the traditional and nontraditional biological functions of angiotensin-converting enzyme. Pharmacol. Rev. 65, 1–46 (2013).
McGregor, R. A. & Choi, M. S. microRNAs in the regulation of adipogenesis and obesity. Curr. Mol. Med. 11, 304–316 (2011).
Hilton, C., Neville, M. J. & Karpe, F. MicroRNAs in adipose tissue: their role in adipogenesis and obesity. Int. J. Obes. (Lond.) 37, 325–332 (2013).
Valenti, M. T., Dalle Carbonare, L. & Mottes, M. Role of microRNAs in progenitor cell commitment and osteogenic differentiation in health and disease (review). Int. J. Mol. Med. 41, 2441–2449 (2018).
Zaragosi, L. E. et al. Small RNA sequencing reveals miR-642a-3p as a novel adipocyte-specific microRNA and miR-30 as a key regulator of human adipogenesis. Genome Biol. 12, R64 (2011).
Chen, S. Z. et al. The miR-181d-regulated metalloproteinase Adamts1 enzymatically impairs adipogenesis via ECM remodeling. Cell Death Differ. 23, 1778–1791 (2016).
Liu, W. et al. LncRNA Gm15290 sponges miR-27b to promote PPARgamma-induced fat deposition and contribute to body weight gain in mice. Biochem. Biophys. Res. Commun. 493, 1168–1175 (2017).
Karbiener, M. et al. MicroRNA-26 family is required for human adipogenesis and drives characteristics of brown adipocytes. Stem Cells 32, 1578–1590 (2014).
Engin, A. B. MicroRNA and adipogenesis. Adv. Exp. Med. Biol. 960, 489–509 (2017).
Belarbi, Y. et al. MicroRNAs-361-5p and miR-574-5p associate with human adipose morphology and regulate EBF1 expression in white adipose tissue. Mol. Cell. Endocrinol. 472, 50–56 (2017).
Fatima, F. & Nawaz, M. Long distance metabolic regulation through adipose-derived circulating exosomal miRNAs: a trail for RNA-based therapies? Front. Physiol. 8, 545 (2017).
Arner, P. & Kulyte, A. MicroRNA regulatory networks in human adipose tissue and obesity. Nat. Rev. Endocrinol. 11, 276–288 (2015).
Arner, E. et al. Adipose tissue microRNAs as regulators of CCL2 production in human obesity. Diabetes 61, 1986–1993 (2012).
Zhu, L. et al. MiR-335, an adipogenesis-related microRNA, is involved in adipose tissue inflammation. Cell Biochem. Biophys. 68, 283–290 (2014).
Ortega, F. J. et al. Surgery-induced weight loss is associated with the downregulation of genes targeted by MicroRNAs in adipose tissue. J. Clin. Endocrinol. Metab. 100, E1467–E1476 (2015).
Schroeder, M., Drori, Y., Ben-Efraim, Y. J. & Chen, A. Hypothalamic miR-219 regulates individual metabolic differences in response to diet-induced weight cycling. Mol. Metab. 9, 176–186 (2018).
Bollepalli, S. et al. Subcutaneous adipose tissue gene expression and DNA methylation respond to both short- and long-term weight loss. Int. J. Obes. (Lond.) 42, 412–423 (2018).
Martinez, J. A., Milagro, F. I., Claycombe, K. J. & Schalinske, K. L. Epigenetics in adipose tissue, obesity, weight loss, and diabetes. Adv. Nutr. 5, 71–81 (2014).
Maurizi, G., Della Guardia, L., Maurizi, A. & Poloni, A. Adipocytes properties and crosstalk with immune system in obesity-related inflammation. J. Cell. Physiol. 233, 88–97 (2018).
Capel, F. et al. Contribution of energy restriction and macronutrient composition to changes in adipose tissue gene expression during dietary weight-loss programs in obese women. J. Clin. Endocrinol. Metab. 93, 4315–4322 (2008).
Vink, R. G. et al. Adipose tissue gene expression is differentially regulated with different rates of weight loss in overweight and obese humans. Int. J. Obes. (Lond.) 41, 309–316 (2017).
Capel, F. et al. Macrophages and adipocytes in human obesity: adipose tissue gene expression and insulin sensitivity during calorie restriction and weight stabilization. Diabetes 58, 1558–1567 (2009).
Schmitz, J. et al. Obesogenic memory can confer long-term increases in adipose tissue but not liver inflammation and insulin resistance after weight loss. Mol. Metab. 5, 328–339 (2016).
Snel, M. et al. Immediate and long-term effects of addition of exercise to a 16-week very low calorie diet on low-grade inflammation in obese, insulin-dependent type 2 diabetic patients. Food Chem. Toxicol. 49, 3104–3111 (2011).
Malisova, L. et al. Expression of inflammation-related genes in gluteal and abdominal subcutaneous adipose tissue during weight-reducing dietary intervention in obese women. Physiol. Res. 63, 73–82 (2014).
Zou, J. et al. CD4+T cells memorize obesity and promote weight regain. Cell. Mol. Immunol. 15, 630–639 (2017).
Kong, L. C. et al. Insulin resistance and inflammation predict kinetic body weight changes in response to dietary weight loss and maintenance in overweight and obese subjects by using a Bayesian network approach. Am. J. Clin. Nutr. 98, 1385–1394 (2013).
Wang, H. & Ye, J. Regulation of energy balance by inflammation: common theme in physiology and pathology. Rev. Endocr. Metab. Disord. 16, 47–54 (2015).
van den Berg, S. M., van Dam, A. D., Rensen, P. C., de Winther, M. P. & Lutgens, E. Immune modulation of brown(ing) adipose tissue in obesity. Endocr. Rev. 38, 46–68 (2017).
Armenise, C. et al. Transcriptome profiling from adipose tissue during a low-calorie diet reveals predictors of weight and glycemic outcomes in obese, nondiabetic subjects. Am. J. Clin. Nutr. 106, 736–746 (2017).
Sumithran, P., Purcell, K., Kuyruk, S., Proietto, J. & Prendergast, L. A. Combining biological and psychosocial baseline variables did not improve prediction of outcome of a very-low-energy diet in a clinic referral population. Clin. Obes. 8, 30–38 (2018).
Caires, R. et al. Omega-3 fatty acids modulate TRPV4 function through plasma membrane remodeling. Cell Rep. 21, 246–258 (2017).
Sidossis, L. & Kajimura, S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J. Clin. Invest. 125, 478–486 (2015).
Shen, W. & McIntosh, M. K. Nutrient regulation: conjugated linoleic acid’s inflammatory and browning properties in adipose tissue. Annu. Rev. Nutr. 36, 183–210 (2016).
Tsiloulis, T. et al. No evidence of white adipocyte browning after endurance exercise training in obese men. Int. J. Obes. (Lond.) 42, 721–727 (2017).
Norheim, F. et al. The effects of acute and chronic exercise on PGC-1alpha, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 281, 739–749 (2014).
Nakhuda, A. et al. Biomarkers of browning of white adipose tissue and their regulation during exercise- and diet-induced weight loss. Am. J. Clin. Nutr. 104, 557–565 (2016).
Pino, M. F., Parsons, S. A., Smith, S. R. & Sparks, L. M. Active individuals have high mitochondrial content and oxidative markers in their abdominal subcutaneous adipose tissue. Obesity (Silver Spring) 24, 2467–2470 (2016).
Steig, A. J. et al. Exercise reduces appetite and traffics excess nutrients away from energetically efficient pathways of lipid deposition during the early stages of weight regain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R656–R667 (2011).
Giles, E. D. et al. Exercise decreases lipogenic gene expression in adipose tissue and alters adipocyte cellularity during weight regain after weight loss. Frontiers Physiol. 7, 32 (2016).
Bartus, R. T. et al. β2-adrenoceptor agonists as novel, safe and potentially effective therapies for amyotrophic lateral sclerosis (ALS). Neurobiol. Dis. 85, 11–24 (2016).
Esser, N., Paquot, N. & Scheen, A. J. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin. Investig. Drugs 24, 283–307 (2015).
Poulsen, M. M. et al. Resveratrol and inflammation: challenges in translating pre-clinical findings to improved patient outcomes. Biochim. Biophys. Acta 1852, 1124–1136 (2015).
Gleeson, M. et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 11, 607–615 (2011).
Lancaster, G. I. & Febbraio, M. A. The immunomodulating role of exercise in metabolic disease. Trends Immunol. 35, 262–269 (2014).
Auerbach, P. et al. Differential effects of endurance training and weight loss on plasma adiponectin multimers and adipose tissue macrophages in younger, moderately overweight men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305, R490–R498 (2013).
van Baak, M. A. et al. Leisure-time activity is an important determinant of long-term weight maintenance after weight loss in the Sibutramine Trial on Obesity Reduction and Maintenance (STORM trial). Am. J. Clin. Nutr. 78, 209–214 (2003).
Kerns, J. C. et al. Increased physical activity associated with less weight regain six years after “the biggest loser” competition. Obesity (Silver Spring) 25, 1838–1843 (2017).
Ostendorf, D. M. et al. Objectively measured physical activity and sedentary behavior in successful weight loss maintainers. Obesity (Silver Spring) 26, 53–60 (2018).
de Luis, D. A. et al. Biochemical, anthropometric and lifestyle factors related with weight maintenance after weight loss secondary to a hypocaloric mediterranean diet. Ann. Nutr. Metab. 71, 217–223 (2017).
Kjaer, T. N. et al. Resveratrol reduces the levels of circulating androgen precursors but has no effect on, testosterone, dihydrotestosterone, PSA levels or prostate volume. A 4-month randomised trial in middle-aged men. Prostate 75, 1255–1263 (2015).
Calder, P. C. Long-chain fatty acids and inflammation. Proc. Nutr. Soc. 71, 284–289 (2012).
Shivappa, N. et al. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br. J. Nutr. 113, 665–671 (2015).
Shivappa, N., Steck, S. E., Hurley, T. G., Hussey, J. R. & Hebert, J. R. Designing and developing a literature-derived, population-based dietary inflammatory index. Publ. Health Nutr. 17, 1689–1696 (2014).
Ramallal, R. et al. Inflammatory potential of diet, weight gain, and incidence of overweight/obesity: the SUN Cohort. Obesity 25, 997–1005 (2017).
Muhammad, H. F. L. et al. Dietary intake after weight loss and the risk of weight regain: macronutrient composition and inflammatory properties of the diet. Nutrients 9, 1205 (2017).
Ravussin, E. et al. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring) 17, 1736–1743 (2009).
Aller, E. E. et al. Weight loss maintenance in overweight subjects on ad libitum diets with high or low protein content and glycemic index: the DIOGENES trial 12-month results. Int. J. Obes. (Lond.) 38, 1511–1517 (2014).
Johansson, K., Neovius, M. & Hemmingsson, E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very-low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 99, 14–23 (2014).
The STORM Study Group. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. Lancet 356, 2119–2125 (2000).
Richelsen, B. et al. Effect of orlistat on weight regain and cardiovascular risk factors following a very-low-energy diet in abdominally obese patients: a 3-year randomized, placebo-controlled study. Diabetes Care 30, 27–32 (2007).
Wadden, T. A. et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int. J. Obes. (Lond.) 37, 1443–1451 (2013).
Vazquez, C. et al. Meal replacement with a low-calorie diet formula in weight loss maintenance after weight loss induction with diet alone. Eur. J. Clin. Nutr. 63, 1226–1232 (2009).
Westerterp-Plantenga, M. S., Lejeune, M. P. & Kovacs, E. M. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes. Res. 13, 1195–1204 (2005).
Dutton, G. R. et al. Comparison of an alternative schedule of extended care contacts to a self-directed control: a randomized trial of weight loss maintenance. Int. J. Behav. Nutr. Phys. Act. 14, 107 (2017).
Voils, C. I. et al. Maintenance of weight loss after initiation of nutrition training: a randomized trial. Ann. Intern. Med. 166, 463–471 (2017).
Crain, A. L., Sherwood, N. E., Martinson, B. C. & Jeffery, R. W. Mediators of weight loss maintenance in the Keep It Off trial. Ann. Behav. Med. 52, 9–18 (2017).
Ryan, A. S., Serra, M. C. & Goldberg, A. P. Metabolic benefits of prior weight loss with and without exercise on subsequent 6-month weight regain. Obesity (Silver Spring) 26, 37–44 (2018).
Wong, M. H. et al. Caloric restriction induces changes in insulin and body weight measurements that are inversely associated with subsequent weight regain. PLOS ONE 7, e42858 (2012).
Hanvold, S. E. et al. Plasma amino acids, adiposity, and weight change after gastric bypass surgery: are amino acids associated with weight regain? Eur. J. Nutr. 57, 2629–2637 (2017).
Sawamoto, R. et al. Predictors of successful long-term weight loss maintenance: a two-year follow-up. Biopsychosoc. Med. 11, 14 (2017).
Calugi, S., Marchesini, G., El Ghoch, M., Gavasso, I. & Dalle Grave, R. The influence of weight-loss expectations on weight loss and of weight-loss satisfaction on weight maintenance in severe obesity. J. Acad. Nutr. Diet 117, 32–38 (2017).
Greenberg, I., Stampfer, M. J., Schwarzfuchs, D., Shai, I. & Group, D. Adherence and success in long-term weight loss diets: the dietary intervention randomized controlled trial (DIRECT). J. Am. Coll. Nutr. 28, 159–168 (2009).
Vogels, N. & Westerterp-Plantenga, M. S. Categorical strategies based on subject characteristics of dietary restraint and physical activity, for weight maintenance. Int. J. Obes. (Lond.) 29, 849–857 (2005).
Fabricatore, A. N. et al. Predictors of attrition and weight loss success: results from a randomized controlled trial. Behav. Res. Ther. 47, 685–691 (2009).
Lillis, J. et al. Weight loss intervention for individuals with high internal disinhibition: design of the Acceptance Based Behavioral Intervention (ABBI) randomized controlled trial. BMC Psychol. 3, 17 (2015).
Butryn, M. L., Thomas, J. G. & Lowe, M. R. Reductions in internal disinhibition during weight loss predict better weight loss maintenance. Obesity (Silver Spring) 17, 1101–1103 (2009).
Abu Dayyeh, B. K., Jirapinyo, P. & Thompson, C. C. Plasma ghrelin levels and weight regain after Roux-en-Y gastric bypass surgery. Obes. Surg. 27, 1031–1036 (2017).
Brock, D. W. et al. Perception of exercise difficulty predicts weight regain in formerly overweight women. Obesity (Silver Spring) 18, 982–986 (2010).
Price, D. W. et al. Depression as a predictor of weight regain among successful weight losers in the diabetes prevention program. Diabetes Care 36, 216–221 (2013).
Larsen, L. H. et al. Analyses of single nucleotide polymorphisms in selected nutrient-sensitive genes in weight-regain prevention: the DIOGENES study. Am. J. Clin. Nutr. 95, 1254–1260 (2012).
Nicklas, B. J. et al. Genetic variation in the peroxisome proliferator-activated receptor-gamma2 gene (Pro12Ala) affects metabolic responses to weight loss and subsequent weight regain. Diabetes 50, 2172–2176 (2001).
McCaffery, J. M. et al. FTO predicts weight regain in the Look AHEAD clinical trial. Int. J. Obes. (Lond.) 37, 1545–1552 (2013).
Delahanty, L. M. et al. Genetic predictors of weight loss and weight regain after intensive lifestyle modification, metformin treatment, or standard care in the Diabetes Prevention Program. Diabetes Care 35, 363–366 (2012).
Masuo, K. et al. Rebound weight gain as associated with high plasma norepinephrine levels that are mediated through polymorphisms in the β2-adrenoceptor. Am. J. Hypertens. 18, 1508–1516 (2005).
Crujeiras, A. B. et al. Association of weight regain with specific methylation levels in the NPY and POMC promoters in leukocytes of obese men: a translational study. Regul. Pept. 186, 1–6 (2013).
Reviewer information
Nature Reviews Endocrinology thanks José Fernandez-Real and other anonymous reviewers for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Energy balance
-
The balance between energy intake and energy expenditure.
- Fasting fatty acid rate of appearance
-
The rate of release of fattyacids into the plasma in thefasting state, which can bedetermined by measuring thedilution of an infused fatty acidtracer in plasma.
- Weight cycling
-
The repeated loss and regain of body weight.
- Bayesian analysis
-
A statistical paradigm that answers research questions about unknown parameters using probability statements.
Rights and permissions
About this article
Cite this article
van Baak, M.A., Mariman, E.C.M. Mechanisms of weight regain after weight loss — the role of adipose tissue. Nat Rev Endocrinol 15, 274–287 (2019). https://doi.org/10.1038/s41574-018-0148-4
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-018-0148-4
This article is cited by
-
Pharmacotherapy for obesity: moving towards efficacy improvement
Diabetology & Metabolic Syndrome (2024)
-
High Prevalence of Positive Genetic Obesity Variants in Postoperative Bariatric Surgery Patients with Weight Regain Presenting for Medical Obesity Intervention
Obesity Surgery (2024)
-
Resistant starch intake facilitates weight loss in humans by reshaping the gut microbiota
Nature Metabolism (2024)
-
Adipocyte p53 coordinates the response to intermittent fasting by regulating adipose tissue immune cell landscape
Nature Communications (2024)
-
Obesity-induced and weight-loss-induced physiological factors affecting weight regain
Nature Reviews Endocrinology (2023)