Abstract
The systemic inflammatory response is known to be associated with poor outcomes in patients with various types of cancer. The C-reactive protein (CRP)/albumin (Alb) ratio (CAR) has been reported as a novel inflammation-based prognostic marker. We have evaluated the prognostic value of inflammatory markers for patients with oral squamous cell carcinoma (OSCC). The study population included 205 patients treated with OSCC between 2013 and 2018. The primary predictor variable was the inflammatory markers. The primary outcome variable was overall survival (OS). Univariate and multivariate analyses were performed using a Cox proportional hazards model to identify independent prognostic factors. The CAR had the highest area under the curve (AUC) values compared with other markers in the receiver operating characteristic (ROC) curve analysis. The cutoff value for CAR was 0.032 (AUC 0.693, P < 0.001). There was a significant difference in OS when patients were stratified according to CAR, with 79.1% for CAR < 0.032 and 35% for CAR ≥ 0.032 (P < 0.001). Cox multivariate analysis identified independent predictive factors for OS: age (hazard ratio [HR] 2.155, 95% confidence interval [CI] 1.262–3.682; P = 0.005), stage (HR 3.031, 95% CI 1.576–5.827; P = 0.001), and CAR (HR 2.859, 95% CI 1.667–4.904; P < 0.001). CAR (≥ 0.032 vs. < 0.032) is a good prognostic marker in patients with OSCC in terms of age and stage.
Similar content being viewed by others
Introduction
Host and tumor factors interact, and these interactions can either accelerate tumor progression or regression. The systemic inflammatory response is associated with poor outcomes in patients diagnosed with various malignancies1. Several common inflammation-based markers, including the neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), lymphocyte/monocyte ratio (LMR), systemic inflammation response index (SIRI), and systemic immune-inflammation index (SII), have been reported previously2,3,4,5.
The C-reactive protein (CRP)/albumin (Alb) ratio (CAR) has been identified as a novel inflammation-based prognostic marker in several cancers, including esophageal cancer, lung cancer, hypopharyngeal and laryngeal cancer, and nasopharyngeal cancer6,7,8,9. CAR is an independent marker of inflammation in various cancers and a more accurate prognostic marker than other markers, such as the modified Glasgow prognostic score (mGPS), NLR, and PLR10,11,12. These inflammation-based prognostic scores can be easily and routinely measured and serve as a valuable prognostic parameter.
To the best of our knowledge, only two reports examining the prognostic value of CAR in oral squamous cell carcinoma (OSCC) have been published13,14. Therefore, the present study aimed to evaluate and compare the prognostic value of inflammatory markers, including the NLR, SIRI, SII, LMR, and the novel prognostic factor, CAR, in patients with OSCC.
Results
Optimal cutoff values of the inflammation-based prognostic scores
The cutoff values for predicting OS were calculated by the receiver operating characteristic (ROC) curve analysis, which determined that the optimal cutoff values for NLR, SIRI, SII, LMR, and CAR were 3.59 (area under the curve [AUC], 0.628; sensitivity, 40.7%; specificity, 81.5%; P = 0.004), 1.19 (AUC, 0.622; sensitivity, 45.8%; specificity, 77.4%; P = 0.006), 823.1 (AUC, 0.598; sensitivity, 40.7%; specificity, 81.5%; P = 0.028), 5.00 (AUC, 0.599; sensitivity, 69.5%; specificity, 52.7%; P = 0.026), and 0.032 (AUC, 0.693; sensitivity, 59.3%; specificity, 75.3%; P < 0.001), respectively. CAR had the highest AUC value (Fig. 1).
Association between patient characteristics and the CAR cutoff value
Based on the CAR cutoff value, the patients were subdivided into two groups: 134 patients presented with a low CAR (< 0.032) and 71 had a high CAR (≧ 0.032). The median age was 71.3 (range: 31.1–93.0) years. Age differed significantly between the two groups (P = 0.007). The patients included 123 men and 82 women with a median pretreatment BMI of 22.49 (range: 13.51–34.21) kg/m2. The most common primary tumor sites included the tongue (n = 76), mandibular gingiva (n = 65), and buccal mucosa (n = 22). There was a significant difference in the primary site between the two groups (P = 0.003). Although there was no significant difference in the mandibular gingiva between the two groups, a significant difference occurred in the tongue vs. others between the two groups (P = 0.002). The TNM classification and stage details are shown in Table 1. There were significant differences in TN classification and stage between the low and high CAR groups (P < 0.01). Management involved only surgery in 89 patients with a low CAR and 27 patients with a high CAR, surgery with or without radiotherapy or chemoradiotherapy in 36 patients with a low CAR and 15 patients with a high CAR, and only radiotherapy in 9 patients with a low CAR and 29 patients with a high CAR. There was a significant difference in the management between the three groups (P < 0.001). The other primary predictor variables that were analyzed in addition to CAR included NLR, SIRI, SII, and LMR. These variables were dichotomized according to predetermined cutoff values, and similar to CAR, all demonstrated significant differences between the two groups (P < 0.001). The results of the other patient-related characteristics/variables are presented in Table 1.
Of the 205 patients with OSCC, 146 (71.2%) survived during the follow-up period; among these, OSCC recurred locally in 39 patients (19.0%), spread regionally in 15 (7.3%), and metastasized distantly in 20 (9.8%). Of the 59 patients (28.8%) who died during the follow-up period, 52 died with OSCC and 7 died without any OSCC recurrence or metastasis. Local recurrence occurred in 15 (38.5%) patients with a low CAR and 24 (61.5%) with a high CAR, and there was a significant difference between the two groups (P < 0.001) (Table 1).
Association between clinical factors and OS
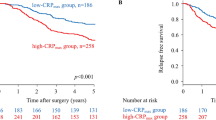
The median follow-up period of the survivors was 45.8 (1.4–87.8) months. The associations between the study variables and OS are presented in Table 2. There were significant differences in OS when patients were stratified according to stage classification (OS rates: stage I, 88.0%; stage II, 71.5%; stage III, 77.4%; stage IVA, 49.4%; stage IVB, 44.9%; and stage IVC, 0%; P = 0.001; Supplementary Fig. 1). There were significant differences in OS with age and TNM classification (P < 0.01). In the primary site, there was a significant difference in OS when patients stratified the tongue and others (OS rates: tongue 79.9% vs. others 55.8%; P = 0.007). Likewise, significant differences in the primary outcome variable (OS) were obtained when patients were stratified according to the primary predictor variable (CAR), with an OS rate of 79.1% for CAR < 0.032 and 35% for CAR ≥ 0.032 (P < 0.001; Fig. 2A). There were significant differences in the OS curve when patients were divided by NLR < 3.59 (70.0%) vs. NLR ≥ 3.59 (50.4%; P < 0.001), SIRI < 1.19 (71.7%) vs. SIRI ≥ 1.19 (47.7%; P = 0.001), SII < 823.1 (70.3%) vs. SII ≥ 823.1 (50.1%; P < 0.001), and LMR ≥ 5.0 (75.2%) vs. LMR < 5.0 (56.1%; P < 0.001) (Fig. 2B–E).
(A) Kaplan–Meier survival curve according to CAR. Patients were stratified according to the cutoff value of CAR (0.032). The OS rate was 79.1% for patients with a low CAR (< 0.032) and 35% for a high CAR (≥ 0.032) (P < 0.001). (B)–(E) Kaplan–Meier survival curves according to NLR, SIRI, SII, and LMR. Significant differences in OS were noted when the patient cohort was subdivided into two groups according to the cutoff value of each inflammatory marker (NLR < 3.59, 70.0% vs. NLR ≥ 3.59, 50.4%, P < 0.001; SIRI < 1.19, 71.7% vs. SIRI ≥ 1.19, 47.7%, P = 0.001; SII < 823.1, 70.3% vs. SII ≥ 823.1, 50.1%, P < 0.001; and LMR ≥ 5.0, 75.2% vs. LMR < 5.0, 56.1%, P < 0.001). A less favorable prognosis was associated with a higher NLR, SIRI, and SII values and a lower LMR value.
In the Kaplan–Meier analysis and the cumulative survival curves of patients stratified into quartiles on the basis of the CAR value range, there were significant differences in OS when patients were stratified into quartiles according to the CAR range (OS rates: CAR < 0.0073, 86.6%; 0.0073 ≤ CAR < 0.0167, 74.9%; 0.0167 ≤ CAR < 0.0569, 50.3%; 0.0569 ≤ CAR, 47.0%; P < 0.001).
Subgroup analysis with management of the surgery group
In 167 patients (81.5%) in the surgery cohort, univariate analyses showed that OS was significantly associated with CAR (≥ 0.032 vs. < 0.032), with a hazard ratio [HR] of 2.262 and 95% confidence interval [CI] of 1.087–4.705 (P = 0.029). Significant associations of OS with NLR (≥ 3.59 vs. < 3.59; HR 2.181, 95% CI 1.021–4.662; P = 0.044) and SII (≥ 823.1vs. < 823.1; HR 2.623, 95% CI 1.262–5.449; P = 0.010) were also identified. As postoperative risk factors, extra nodal extension (ENE) and positive LN counts (< 2 or ≥ 2) were analyzed, showing that OS was significantly associated with ENE (present vs. absent; HR 9.360, 95% CI 3.724–23.526; P < 0.001) and LN counts (< 2 or ≥ 2; HR 3.043, 95% CI 1.423–6.507; P < 0.004) (Table 3).
Subgroup analysis according to stage and CAR
A subgroup analysis was performed to evaluate the prognostic value of CAR when patients were stratified by stage and age. The OS results obtained for patients when grouped according to both CAR (< 0.032 or ≥ 0.032), stage (I, II or III, IV), and age (≥ 71.3 vs. < 71.3) are depicted in Fig. 3. There were significant differences in OS when patients were stratified by a combination of CAR, stage, and age (OS rates: CAR <, stage <, age <, 91.3%; CAR <, stage <, age ≥, 85.3%; CAR <, stage ≥, age <, 76.0%; CAR ≥, stage <, age ≥, 64.3%; CAR ≥, stage ≥, age <, 63.1%; CAR <, stage ≥, age ≥ 58.0%; CAR ≥, stage ≥, age ≥, 19.7%; CAR ≥, stage <, age <, 0%; P < 0.001). The combination of CAR <, stage <, and age < had the most favorable OS rate, while CAR ≥, stage ≥, age ≥, and CAR ≥, stage <, and age < had the least favorable prognosis.
Kaplan–Meier survival curve according to a combination of CAR (< 0.032 or ≥ 0.032), stage (I, II or III, IV), and age (< 71.3 or ≥ 71.3). There were significant differences in OS when the patient cohort was stratified according to a combination of CAR and stage (OS rates: CAR <, stage <, age <, 91.3%; CAR <, stage <, age ≥, 85.3%; CAR <, stage ≥, age <, 76.0%; CAR ≥, stage <, age ≥, 64.3%; CAR ≥, stage ≥, age <, 63.1%; CAR <, stage ≥, age ≥ 58.0%; CAR ≥, stage ≥, age ≥, 19.7%; CAR ≥, stage <, age <, 0%; P < 0.001).
Cox multivariate regression analysis and logistic multivariate analysis
Univariate analyses showed that OS was significantly associated with CAR (≥ 0.032 vs. < 0.032), with an HR of 3.839 and 95% CI of 2.275–6.476 (P < 0.001). Significant associations between OS and age (≥ 71.3 vs. < 71.3; HR 2.077; 95% CI 1.224–3.527; P = 0.007), T classification (T1, 2 vs. T3, 4; HR 3.160; 95% CI 1.779–5.613; P < 0.001), N classification (N0, 1 vs. N2, 3; HR 2.285, 95% CI 1.347–3.878; P = 0.002), stage (I, II vs. III, IV; HR 3.617; 95% CI 1.918–6.824; P < 0.001), NLR (≥ 3.59 vs. < 3.59; HR 2.681; 95% CI 1.593–4.512; P < 0.001), SIRI (≥ 1.19 vs. < 1.19; HR 2.423; 95% CI 1.447–4.059; P = 0.001), SII (≥ 823.1vs. < 823.1; HR 2.578; 95% CI 1.532–4.340; P < 0.001), and LMR (≥ 5.0 vs. < 5.0; HR 0.380; 95% CI 0.218–0.663; P = 0.001) were also identified (Table 4).
Logistic multivariate analysis of the parameters with stepwise forward selection method identified three independent predictive factors for survival: age (≥ 71.3 vs. < 71.3) (odds ratio [OR], 0.466; 95% CI, 0.235 to 0.924; P = 0.029), stage (I, II vs. III, IV) (OR, 0.269; 95% CI, 0.125 to 0.578; P = 0.001), and CAR (OR, 0.328; 95% CI, 0.166 to 0.648; P = 0.001); details are shown in Supplementary Table 1. In the Cox multivariate analysis, the parameters with significant variables by stepwise forward selection identified three independent predictive factors for OS: age (HR 2.155, 95% CI 1.262–3.682; P = 0.005), stage (HR 3.031, 95% CI 1.576–5.827; P = 0.001), and CAR (HR 2.859, 95% CI 1.667–4.904; P < 0.001). These results indicate that, of the variables analyzed in this study, age (≥ 71.3 vs. < 71.3), stage (I, II vs. III, IV), and CAR (≥ 0.032 vs. < 0.032) were more useful markers for predicting prognosis (Table 4).
Discussion
The systemic inflammatory response influences carcinogenesis and cell proliferation, tumor cell migration, invasion, metastasis, cell survival, angiogenesis15,16. Kinoshita et al. demonstrated that CAR can serve as a novel inflammation-based prognostic score to predict survival in hepatocellular carcinoma12. The inflammatory marker, CRP, and nutritional marker, serum Alb, are widely used clinically and inexpensive to evaluate17,18. The CAR value provides an indication of the serum CRP concentrations relative to the serum concentration of Alb. A high CRP score may indicate an elevated serum CRP concentration in conjunction with hypoalbuminemia, an elevated CRP concentration relative to normal Alb levels, or normal CRP concentrations relative to a depressed Alb concentration. However, it has been reported that CAR may prove more useful in assessing disease status and predicting the long-term outcomes of malignancy19.
Secretion of pro-inflammatory cytokines, particularly interleukin 1 (IL-1) and 6 (IL-6), and chemokines, such as NF-κB, by tumor cells and tumor-infiltrating lymphocytes stimulates the immune and hematopoietic systems and upregulates the production of CRP, neutrophils, lymphocytes, platelets, and so on20. Serum CRP is produced mainly by hepatocytes, with production regulated by proinflammatory cytokines, especially IL-6. Therefore, CRP levels are indicative of tumor activity19. In the present study, a significant difference in OS between patients with high (≥ 0.105) and low CRP (< 0.105) levels was identified, with high CRP levels associated with a poorer prognosis. This result is consistent with the findings of previous reports.
Serum Alb is used as an indicator of nutritional status. Low levels are associated with poor survival outcomes in various cancers, including head and neck cancer (HANC)17,18,19,21. In the present study, a significant difference in OS between high (≥ 4.15) and low Alb (< 4.15) concentrations was noted, with hypoalbuminemia related to a significantly poor prognosis. Crumley et al. reported on the association between CRP and Alb and concluded that the relationship between hypoalbuminemia and poor survival was secondary to that of the systemic inflammatory response21. Similarly, according to Liu et al., prognosis was not significantly associated with CAR and preoperative BMI. In terms of CAR, the systemic inflammatory response exerted a more potent prognostic effect than nutritional status22. In the present patient cohort, there was no association between CAR and preoperative BMI, which corresponded with the suggestion that hypoalbuminemia and poor survival are secondary to the systemic inflammatory response.
Increased NLR has been found to be significantly associated with poor OS in patients with laryngeal cancer23. Rassouli et al. demonstrated that elevated PLR was correlated with higher mortality in HANC, suggesting that NLR and PLR as combined cellular components of systemic inflammation have a potential value for predicting cancer-specific survival in HANC24. In the present study, there were significant differences in OS between the two patient groups divided according to the cutoff values of NLR, SIRI, SII, LMR, and CAR. Moreover, the univariate Cox regression analysis showed significant differences in NLR, SIRI, SII, LMR, and CAR. However, it has been reported that CAR is superior to other inflammation-based prognostic scores, including NLR and PLR because CAR was found to have a higher AUC value than that obtained for the other markers1,22. Similarly, in the present study, the AUC value calculated for CAR on ROC curve analysis was found to be the highest. Moreover, on multivariate analysis, CAR, age, and stage were selected as independent predictors of OS. Therefore, CAR is currently recognized as the most useful prognostic marker. Furthermore, on Kaplan–Meier analysis, significant differences in OS were observed between groups when patients were stratified into quartiles, according to the CAR value range.
Table 5 summarizes the previous studies that have reported on the prognostic utility of CAR in various malignancies1,5,8,10,12,13,14,19,20,22,25,26. The cutoff values of CAR for the different cancers ranged from 0.023 to 0.525. Of the 13 studies, 5 (38.4%) were concerned with esophageal cancers, while only 3 (23.1%), including the present paper, related to OSCC. In HANC, excluding OSCC and including meta-analyses, three articles elucidating the prognostic value of CAR in nasopharyngeal cancer9,25,27 and one in hypopharyngeal cancer have been published8. Although the present study also demonstrated that CAR may be a more sensitive prognostic predictor in OSCC, the optimal predictive cutoff value differed from previously reported. Instead, a wide range of cutoff values were observed across the studies. This suggests that the significant CAR cutoff value may differ with the cancer type, pathological type, clinical stage, and type of treatment, and further research will be required to confirm this suspicion. In the previous two OSCC reports, the cutoff value of Wang et al. is 0.525, which is the highest of all CAR reports13. This value may be due to the different methods used to determine the cutoff value by Cutoff Finder analysis, the difference in the population of patients who had undergone surgery, and a short period of observation within 50 months. In contrast, Park et al. reported a cutoff value of 0.08514. Although this cutoff value is close to our cutoff value, the subjects were only 40 patients. A multiple center and large sample size analysis will be desired to determine the optimal cutoff value in patients with OSCC. In addition, there was a significant difference in the primary site of the tongue vs. others between CAR (≥ 0.032 vs. < 0.032). When patients were stratified based on primary site of the tongue and others, there was a significant difference in OS. This result may be due to the good OS of patients with the tongue as primary site. In the future, subgroup analysis of the primary site with a large sample size will be interesting.
In the subgroup analysis of the surgery group, OS was significantly associated with CAR, NLR, and SII. The cutoff value of CAR 0.032 derived from this study was a useful preoperative predictor for the surgery cohort. Although the recognized postoperative predictive risk factors for OSCC (ENE and positive LN counts ≥ 2) are more significant than CAR, CAR is also a meaningful factor as a preoperative predictor.
The levels of inflammatory components have a certain prognostic value in cancer1. In the present study, Cox multivariate regression analysis identified three independent predictive factors for OS: age, stage, and CAR. These results suggest that the prognosis of OSCC is defined not only by the clinical stage of the tumor and age, but also by systemic host factors. Therefore, a combination analysis with age, clinical stage as the tumor factor, and CAR as the host factor was performed. Significant differences in OS rates were observed between groups when the patients were subdivided according to CAR, age, and tumor stage. The most favorable survival outcomes were obtained for those with a low CAR value (< 0.032), low stage (I or II), and low age (< 71.3), while the opposite was true for those presenting with a high CAR value (≥ 0.032), high stage (III or IV), and high age (≥ 71.3). Although a high CAR value (≥ 0.032), tumor stage I or II, and low age (< 71.3) had the worst survival outcomes, the case count was only 4 and the significance was unknown. In a study that investigated the predictive utility of CAR in esophageal cancer, patients with a high CAR usually experienced severe tumor-related inflammatory reactions or poor nutritional status; these patients may benefit from anti-inflammatory therapy or nutritional support. Anti-inflammatory therapy and nutritional support should be added to the individualized treatment regimen of patients with a high CAR6. In OSCC, a high CAR was found to be associated with a less favorable prognosis. Likewise, those presenting with a high CAR and stage III or IV cancer may require a more aggressive treatment regimen, including radiotherapy and chemotherapy, than indicated by their general condition. Additional long-term studies will be necessary to validate this finding.
As CAR can be readily assessed through routine blood tests, it is a useful, simple, objective, reproducible, and economically feasible prognostic indicator in patients with OSCC. Limitations of the present study include its retrospective, single-institute design and possible selection bias during patient and data collection. In addition, there was considerable heterogeneity in the treatment provided to the patients. Therefore, a long-term prospective multicenter study will be required to validate the preliminary findings of the present report.
In conclusion, among inflammation-based prognostic markers, the AUC was highest for CAR, and in the Cox multivariate analysis, CAR (≥ 0.032 vs. < 0.032), in addition to age and stage, was identified as an independent predictor and, thus, a useful prognostic marker in OSCC. CAR is a novel inflammation-based prognostic marker for patients with OSCC.
Materials and methods
Study design and patients
The present retrospective cohort study included patients diagnosed with OSCC who underwent treatment between 2013 and 2018 at the Department of Oral and Maxillofacial Surgery, University of Tsukuba Hospital, Ibaraki, Japan. From an initial sample size of 259, 54 patients were excluded because they did not undergo treatment and/or palliative therapy. Hence, a total of 205 patients were included in this study. Cancer was staged according to the 2017 Union for International Cancer Control categories (8th edition). The main initial treatments were surgery for patients with resectable tumors in an operable general condition. According to the postoperative pathological results, the high-risk group (ENE +, LN counts ≥ 2, close or positive margin) was treated with adjuvant radiotherapy (60–66 Gy) and/or chemotherapy (CDDP 100 mg/m2, 2 or 3 courses). Salvage therapy was mainly radiotherapy (70 Gy) with chemotherapy (CDDP 100 mg/m2, 2 or 3 courses). The follow-up duration was every 2 to 4 weeks in the first year, 2 months in 2 years, 3 months in 3 years, 4 months in 4 years, and 5 months in 5 years with CT and MRI examination. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the University of Tsukuba Hospital. Informed consent was waived due to the retrospective nature of the study (No. R02-117).
Study variables
The primary predictor variables were inflammation-based markers. The ROC curve, AUC, sensitivity, specificity, and 95% CI were calculated to determine the best-defined risk groups as follows: preoperative blood examination data (NLR, SIRI, SII, LMR, PLR, CAR). The AUC was measured to evaluate and compare the discrimination ability of the variables. The patients were divided into binary subgroups using the best-defined preoperative blood examination data (NLR, SIRI, SII, LMR, PLR, and CAR) as the cutoff point. The cutoff values for predicting OS were determined by ROC curve analysis based on the maximum Youden index. The primary outcome variable was OS, and the other variables were related to patient characteristics, including sex, age, and tumor stage.
Statistical analyses
CAR was selected as the primary predictor variable, as the highest AUC value was calculated for this inflammatory marker relative to the other inflammation-based markers that were examined. The optimal cutoff level for CAR was 0.032 for OS. Patients were divided into high CAR (≥ 0.032) or low CAR (< 0.032) subgroups using this cutoff value, and differences between the subgroups were analyzed for significance. Survival curves were plotted according to the Kaplan–Meier method, and any differences were analyzed using the log-rank test. OS was calculated from the date of first diagnosis to death from any cause. The cutoff date for surviving patients was May 2020. Subgroups were compared using the Mann–Whitney U test and Chi-square test. Univariate and multivariate analyses for OS were performed using a Cox proportional hazards model. Logistic multivariate analysis of the parameters with stepwise forward selection method was used to identify independent variables for multivariate Cox regression analysis. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software version 25 for Macintosh (SPSS, Chicago IL, USA). P < 0.05 was considered statistically significant.
References
Wei, X.-L. et al. A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: The C-reactive protein/albumin ratio. BMC Cancer 15, 1–11 (2015).
Valero, C. et al. Pretreatment peripheral blood leukocytes are independent predictors of survival in oral cavity cancer. Cancer 126, 994–1003 (2020).
Valero, C. et al. Prognostic capacity of systemic inflammation response index (SIRI) in patients with head and neck squamous cell carcinoma. Head Neck 42, 336–343 (2020).
Tham, T. et al. Systemic immune response in squamous cell carcinoma of the head and neck: A comparative concordance index analysis. Eur. Arch. Otorhinolaryngol. 276, 2913–2922 (2019).
Ishibashi, Y. et al. Prognostic value of preoperative systemic immunoinflammatory measures in patients with esophageal cancer. Ann. Surg. Oncol. 25, 3288–3299 (2018).
Liu, Z., Shi, H. & Chen, L. Prognostic role of pre-treatment C-reactive protein/albumin ratio in esophageal cancer: A meta-analysis. BMC Cancer 19, 1161 (2019).
Deng, T.-B., Zhang, J., Zhou, Y.-Z. & Li, W.-M. The prognostic value of C-reactive protein to albumin ratio in patients with lung cancer. Medicine (Baltimore) 97, e13505 (2018).
Kuboki, A. et al. Prognostic value of C-reactive protein/albumin ratio for patients with hypopharyngeal and laryngeal cancer undergoing invasive surgery involving laryngectomy. Head Neck 41, 1342–1350 (2019).
Yang, X. et al. Prognostic value of pretreatment C-reactive protein/albumin ratio in nasopharyngeal carcinoma A meta-analysis of published literature. Medicine (Baltimore) 97, e11574 (2018).
Haruki, K. et al. The C-reactive protein to albumin ratio predicts long-term outcomes in patients with pancreatic cancer after pancreatic resection. World J. Surg. 40, 2254–2260 (2016).
Ishizuka, M. et al. Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann. Surg. Oncol. 23, 900–907 (2016).
Kinoshita, A. et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann. Surg. Oncol. 22, 803–810 (2015).
Wang, Q. et al. Preoperative high c-reactive protein/albumin ratio is a poor prognostic factor of oral squamous cell carcinoma. Future Oncol. 15, 2277–2286 (2019).
Park, H., Kim, M. & Kim, C. C-reactive protein/albumin ratio as prognostic score in oral squamous cell carcinoma. J. Korean Assoc. Oral Maxillofac. Surg. 42, 243–250 (2016).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
Shrotriya, S., Walsh, D., Bennani-Baiti, N., Thomas, S. & Lorton, C. C-reactive protein is an important biomarker for prognosis tumor recurrence and treatment response in adult solid tumors: A systematic review. PLoS ONE 10, e0143080 (2015).
Lim, W. S. et al. Pretreatment albumin level predicts survival in head and neck squamous cell carcinoma. Laryngoscope 127, E437–E442 (2017).
Kudou, K. et al. C-reactive protein/albuminratio is a poor prognostic factor of esophagogastric junction and upper gastric cancer. J. Gastroenterol. Hepatol. 34, 355–363 (2019).
Yu, X. et al. The value of preoperative Glasgow prognostic score and the C-reactive protein to albumin ratio as prognostic factors for long-term survival in pathological T1N0 esophageal squamous cell carcinoma. J. Cancer 9, 807–815 (2018).
Crumley, A. B. C., Stuart, R. C., McKernan, M. & McMillan, D. C. Is hypoalbuminemia an independent prognostic factor in patients with gastric cancer?. World J. Surg. 34, 2393–2398 (2010).
Liu, X. et al. Preoperative C-reactive protein/albumin ratio predicts prognosis of patients after curative resection for gastric cancer. Transl. Oncol. 8, 339–345 (2015).
Wong, B. Y. W., Stafford, N. D., Green, V. L. & Greenman, J. Prognostic value of the neutrophil-to-lymphocyte ratio in patients with laryngeal squamous cell carcinoma. Head Neck 38(Suppl 1), E1903–E1908 (2016).
Rassouli, A., Saliba, J., Castano, R., Hier, M. & Zeitouni, A. G. Systemic inflammatory markers as independent prognosticators of head and neck squamous cell carcinoma. Head Neck 37, 103–110 (2015).
Sun, P. et al. The ratio of C-reactive protein/albumin is a novel inflammatory predictor of overall survival in cisplatin-based treated patients with metastatic nasopharyngeal carcinoma. Dis. Mark. 2017, 6570808 (2017).
Xu, X.-L., Yu, H.-Q., Hu, W., Song, Q. & Mao, W.-M. A novel inflammation-based prognostic score, the C-reactive protein/albumin ratio predicts the prognosis of patients with operable esophageal squamous cell carcinoma. PLoS ONE 10, e0138657 (2015).
Gao, N., Yang, R.-N., Meng, Z. & Wang, W.-H. The prognostic value of C-reactive protein/albumin ratio in nasopharyngeal carcinoma: A meta-analysis. Biosci. Rep. 38, BSR20180686 (2018).
Acknowledgements
We would like to thank Editage (www.editage.com) for the English language editing.
Author information
Authors and Affiliations
Contributions
K.Y. contributed to the study design and data collection. S.F and N.I.K. conducted the clinical examination. F.U. contributed to the data analysis and the creation of figures and tables. K.Y. and B.H. contributed to the writing of the manuscript. All authors were involved in the data interpretation, review, and approval of the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamagata, K., Fukuzawa, S., Ishibashi-Kanno, N. et al. Association between the C-reactive protein/albumin ratio and prognosis in patients with oral squamous cell carcinoma. Sci Rep 11, 5446 (2021). https://doi.org/10.1038/s41598-021-83362-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-83362-2
This article is cited by
-
Prognostic significance of pre- and post-treatment hematological biomarkers in patients with head and neck cancer treated with chemoradiotherapy
Scientific Reports (2023)
-
Utility of prognostic nutritional index and systemic immune-inflammation index in oral cancer treatment
BMC Cancer (2022)
-
Clinical risk factors for severity and prognosis of antiresorptive agent-related osteonecrosis of the jaw: a retrospective observational study
Journal of Bone and Mineral Metabolism (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.