Content
Enteroviruses generally cause mild disease; however, neonates are at higher risk for severe illness because of the immaturity of their immune systems. Neonatal systemic enterovirus disease, characterized by multiorgan involvement, is among the most serious, potentially fatal conditions associated with enterovirus infection. Typical clinical presentations include encephalomyocarditis (characteristic of group B coxsackieviruses) and hemorrhage-hepatitis syndrome (typical of echovirus 11).[1,2] To describe the severity of neonatal illness associated with coxsackievirus B1 (CVB1) infection, CDC analyzed case reports and preliminary data from the National Enterovirus Surveillance System (NESS) for 2007. This report describes the results of that analysis, which indicated that, in 2007, CVB1 for the first time was the predominant enterovirus in the United States, accounting for 113 (25%) of 444 enterovirus infections with known serotypes. In addition, phylogenetic analysis of the 2007 CVB1 strains suggested that the cases resulted from widespread circulation of a single genetic lineage. Health-care providers and public health departments should be vigilant to the possibility of neonatal disease caused by CVB1. Testing for enteroviruses in clinically compatible cases and reporting of identified enteroviruses to NESS should be encouraged.
NESS is a voluntary, passive surveillance system for monitoring enterovirus infections in the United States. Participating laboratories, which include public health and private laboratories and the CDC Picornavirus Laboratory, report enterovirus detections to NESS on a monthly basis. Each report includes age, sex, state, specimen type and collection date, and enterovirus serotype.
Beginning in August 2007, CDC received multiple reports of cases of severe neonatal illness and death associated with enterovirus infection. CVB1 was identified as the causative agent in many of these cases. Previously, no fatal infection of CVB1 had been reported to NESS.[3] On the basis of these reports, CDC began a review of clinical, virologic, and surveillance data related to enterovirus for 2007, in collaboration with local and state public health departments and hospitals. A case of CVB1 infection was defined as detection of enterovirus by reverse transcription-polymerase chain reaction (RT-PCR) or viral culture, with the virus typed as CVB1 by molecular (i.e., RT-PCR sequencing) or antigenic (i.e., neutralization or immunofluorescence) methods.
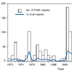
As of February 1, 2008, NESS had received 514 reports of enterovirus infections in 36 states for 2007. CVB1 was the most commonly detected enterovirus reported to NESS, accounting for 113 (25%) of 444 reports with known serotypes (Figure 1). Other most frequently reported serotypes included echovirus 18 (63 [14%]), echovirus 9 (49 [11%]), and echovirus 6 (37 [8%]). Children aged <1 year accounted for 65 (68%) of 95 CVB1 reports with known age, including 50 (53%) infants aged ≤1 month. CVB1 was detected in 19 states; 58% of all CVB1 detections were reported from California (n = 38) and Illinois (n = 28).
Number of reports of coxsackievirus B1 (CVB1) infection and percentage of CVB1 reports among all enterovirus infections with known serotypes -- National Enterovirus Surveillance System, United States, 1970-2007
Phylogenetic analysis of current CVB1 strains based on partial sequence of the VP1 gene revealed that all were closely related to each other and to a 2006 strain from Colorado. Analysis also revealed that the strains were more distantly related to earlier strains.
A total of five CVB1-associated neonatal deaths were identified: two from California, one from Illinois, and one death each from Colorado and New Mexico. These came to CDC attention in connection with requests for laboratory assistance ( Table 1 ). In all five cases, the neonates had multisystem disease with onset within the first 4 days of life. In four of the five fatal cases, the mothers had febrile illness or chorioamnionitis around the time of delivery, suggesting vertical mother-to-infant transmission.
The three distinct clusters of severe enterovirus illness, including illnesses caused by CVB1, detected in Los Angeles, California, Chicago, Illinois, and Kotzebue, Alaska, during 2007 are described below.
In September 2007, in response to reports of three cases (two of them fatal) of neonatal enterovirus myocarditis, including two in CVB1-positive neonates, the Los Angeles County Department of Public Health asked all hospitals in the county to report all enterovirus-positive cases of severe or fatal myocarditis, aseptic meningitis, or sepsis-like febrile illness that occurred among children during June-November 2007.
A total of 30 enterovirus-positive patients from seven hospitals were identified (all with illness diagnosed by RT-PCR). Median age was 15 days (range: <1 day-14 years); 22 (73%) were aged <1 month. Four (13%) patients aged <1-7 days died, and another 14 (47%) required intensive-care unit (ICU) treatment. Clinical presentations included meningitis (22 patients), myocarditis (12), sepsis-like illness (five), hepatitis (two), coagulopathy (six), and respiratory difficulties (three). Eleven patients, including all nine patients aged <7 days at admission, had illness with multiorgan involvement.
Enterovirus serotype was determined in 19 cases for which isolates obtained by viral culture were available. CVB1 accounted for 14 cases; CVB2 accounted for two cases, and CVB3, CVB4, and echoviruses 7 and 11 accounted for one case each. One patient was coinfected with CVB1 and CVB3. Two of the four patients who died were infected with CVB1 ( Table 1 ). Specimens from the other two patients who died were not available for virus characterization.
In September 2007, the CDC Picornavirus Laboratory identified CVB1 as the source of infection in two cases of severe neonatal disease at Children's Memorial Hospital in Chicago. Subsequently, a review of the hospital's laboratory and medical records was conducted to identify additional enterovirus-positive cases and obtain diagnoses and clinical syndrome information. Fifty enterovirus-positive children (all diagnosed by RT-PCR) were admitted during June 6-November 2, 2007, a two-fold increase compared with the entire years 2005 (25 patients) and 2006 (26 patients). Median age of patients was 33 days (range: <1 day-8 years); 40 (80%) patients were aged ≤1 month.
Serotype was determined for nine patients admitted to ICU; CVB1 was found in eight patients, and echovirus 18 in one patient. In two other patients, an enterovirus was identified by immunofluorescence staining as a group B coxsackievirus. Specimens from the remaining 39 RT-PCR-positive patients were not available for further virus characterization.
Twelve (24%) infants aged <1-12 days required ICU admission. Their clinical presentations included myocarditis (11 patients), respiratory distress (nine), hepatitis (eight), coagulopathy (six patients), aseptic meningitis (four), and meningoencephalitis (three). Eleven (92%) patients had multiorgan involvement, including five with myocarditis, meningitis, or meningoencephalitis, and hepatitis (three also had coagulopathy). One CVB1-positive patient died ( Table 1 ), and one required heart transplantation.
In early September 2007, Maniilaq Health Center notified the Alaska Department of Health and Social Services of an increase in severe febrile illness among hospitalized young infants (including three with myocarditis). Medical record review indicated that during August 15-September 11, 2007, seven infants aged ≤1 month (23% of 31 babies born in the Northwest Arctic Borough region since July 1, 2007) had been admitted to the health center with fever and respiratory distress, myocarditis, or meningitis (median age: 18 days; range: 5-48 days). Six patients, five with multiorgan involvement, required ICU treatment, referral to a higher-level hospital, or both. Of these, three patients had myocarditis with aseptic meningitis and respiratory failure (including one with elevated liver enzymes and coagulopathy), two had aseptic meningitis and respiratory distress, and one had aseptic meningitis. The patient with milder illness had a febrile syndrome. None of the patients died.
One patient with myocarditis tested enterovirus-positive by RT-PCR, but the specimen was not available for further virus characterization. CVB1 was isolated from a stool specimen of the patient with aseptic meningitis. The etiologic agent remained unknown in five cases. In addition, CVB1 was isolated from a respiratory specimen of an infant aged 12 months with pneumonia who was treated at the health center as an outpatient during the same period.
Morbidity and Mortality Weekly Report. 2008;57(20):553-556. © 2008 Centers for Disease Control and Prevention (CDC)
Cite this: Increased Detections and Severe Neonatal Disease Associated With Coxsackievirus B1 Infection -- United States, 2007 - Medscape - May 20, 2008.





Comments