Materials and Methods
Collection and Analysis of Bone Marrow
Prior to any therapy, between 10 and 20 ml of bone marrow were aspirated from the anterior iliac crest of 254 primary breast cancer patients undergoing surgical treatment from 2005 to 2007 at the Department of Gynecology and Obstetrics, University Hospital Tuebingen, Germany.
The characteristics of the patients are shown in Table 1 . All specimens were obtained after written informed consent was given and were collected using protocols approved by the institutional review board (114/2006A). Tumour cell isolation and detection was performed based on the recommendations for standardised tumour cell detection.[10] BM samples were separated by density centrifugation over Ficoll with a density of 1.077 g/ml (Biochrom, Germany). If necessary red blood cells were lysed with lysis buffer (155 mM NH4Cl, 10 mM KHC03, 0.1 mM EDTA pH 7.2). Using a cytocentrifuge (Hettich, Tuttlingen, Germany), 106 mononuclear cells were spun onto a glass slide. The slides were air-dried overnight at room temperature. For detection and characterisation of DTCs, slides were fixed in a 0.5% neutral buffered formalin solution for 10 minutes. Control cytospins with ERα-positive MCF-7 cells were prepared, stored and fixed in the same way to ensure that ERα negativity of a patient's sample was not due to a handling error. Two slides per patient was analysed for the presence of DTCs (2 × 106 cells per patient).
Optimising the ERα Staining Protocol
For establishing the ERα staining procedure, preparations of breast cancer cell lines MCF-7 and SKBR3 mixed with either BM or peripheral blood mononuclear cells (PBMCs) from a healthy volunteer were used (Figure 1). To optimise the staining procedure, all relevant parameters of the protocol were evaluated as follows: types of primary ERα antibodies used were monoclonal mouse antibodies (NCL-L-ER-6F11, Novocastra Laboratories, UK), polyclonal rabbit antibodies (H-184, Santa Cruz Biotechnology, Inc., CA) and monoclonal rabbit antibodies (SP1, Lab Vision, CA); antibody dilutions used were 1:200, 1:100, 1:50 and 1:25 made with DAKO Antibody Diluent (1% BSA in PBS, 0.1% Tween 20); incubation times for primary and secondary antibodies were 30, 45 and 60 minutes; selection of secondary antibodies was with Tex-Red labelled horse anti-mouse AB (Vector Laboratories, Inc., CA), Tex-red labelled goat anti-rabbit AB (CB 11, Biogenex, CA) and Alexa Fluor 594 labelled goat anti rabbit AB (Molecular Probes, Invitrogen, CA); cell fixation was 10 minutes of acetone at 4°C, 100% ethanol for 10 minutes or 0.5% neutral buffered formalin solution for 10 minutes, all three fixations at room temperature. The optimal ERα staining (low background, strong nuclear staining, no cytoplasmic staining) was determined to be as indicated below.
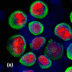
Figure 1.
Oestogen receptor (ER) α staining of MCF-7 (positive control) and SKBR3 (negative control) breast cancer cells spiked in bone marrow. A: MCF-7 cancer cells as positive control for ERα-staining. B: SKBR3 cancer cells as negative control ERα-staining.
Immunofluorescence Staining of ERα-receptor
After an initial washing step with PBS (Sigma, Munich, Germany), cells were blocked for 30 minutes with normal goat serum (Dako, Glostrup, Denmark) at a 1:10 dilution. The automated double immunofluorescence staining procedure was performed on the DAKO Autostainer using the monoclonal rabbit ERα-antibody SP1 (dilution 1:25, Lab Vision, Fremont, CA, USA) for 60 minutes and secondary detection with a goat anti-rabbit antibody, labelled with Alexa Fluor 594 (1:100, Invitrogen Molecular Probes, Carlsbad, CA, USA) for 30 minutes. Cytospins were then incubated with a pan-cytokeratin (CK) antibody (C11) directly conjugated to fluorescein isothiocyanate (FITC) (1:100, Sigma, Munich, Germany) for 30 minutes. This monoclonal antibody recognises human CKs 4, 5, 6, 8, 10, 13 and 18. Counterstaining was performed with 4'6-diamidino-2-phenylindole (DAPI) in mounting media (Vector Laboratories, Burlingame, CA, USA). Preparations of the breast cancer cell line MCF-7 mixed with PBMCs from a healthy volunteer served as a positive control for CK and ERα staining. ERα negative control slides of SKBR-3/PBMC mixtures were also included with each batch of samples. Cytospins of PBMCs with no added tumour cells served as a negative control for both.
Fluorescence Microscopy
Slides were manually analysed for the presence of tumour cells using a computerised fluorescence microscope Axiophot (×40 oil immersion objectives, Carl Zeiss Micro Imaging GmbH, Göttingen, Germany). To screen for ERα-positive tumour cells, a single-pass filter for individual fluorochromes, FITC, Texas Red or DAPI, and a dual-pass filter for FITC/Texas Red were used. Criteria for evaluation of immunostained cells were based on the criteria of the International Society of Hematotherapy and Graft Engineering Working group for standardisation of tumour cell detection and the consensus statements.[10,11] Criteria for ERα positivity were either moderate or intense staining of the entire nucleus. Slides were evaluated by two, or in doubtful cases three, independent investigators (TF, NK and ES).
Immunohistochemical Staining of the Primary Tumour
Immunohistochemical analysis was performed either on core biopsies or surgical resection specimens. The tissue was fixed in 4.5% buffered formalin (pH 7.0) and embedded in paraffin. Immunohistochemical staining was performed on 3 to 5 μm thick sections using a commercially available ABC kit (Vectastain, Vector Laboratories, Burlingame, CA, USA). The ERα antibody (clone SP1) was diluted 1:200 in Tris-HCl (pH 7.5) and applied according to the manufacturer's instruction (DCS, Hamburg, Germany). 3,3'diaminobenzidine (DAB) was used as a chromogen. Finally, the slides were counterstained with haematoxylin and mounted for examination. For assessment of the ERα status, the percentage of cells with nuclear reactivity (score 0: none, 1: > 10%, 2: 10 to 50%, 3: 51 to 80%, 4: > 80%) and the intensity of ER staining (score 0: none, 1: weak, 2: moderate, 3: strong) was determined. ERα expression was scored semi-quantitatively using the Remmele-score (score nuclear staining × score intensity of ER staining). Tumours with a score of 2 or more were considered ERα positive.
Statistical Analysis
A chi-squared test or Fisher's exact test was used to evaluate the relation between ERα-positive DTCs and clinicopathological factors. Statistical analysis was performed by SPSS, version 11.5 (SPSS Inc., Chicago, IL, USA). p < 0.05 was considered statistically significant.
Breast Cancer Res. 2008;10(5) © 2008 BioMed Central, Ltd.
Copyright to this article is held by the author(s), licensee BioMed Central Ltd. This is an Open Access article: verbatim copying and redistribution of this article are permitted in all media for any purpose, provided this notice is preserved along with the article's original citation.
Cite this: ER Alpha-Status of Disseminated Tumor Cells in Bone Marrow of Primary Breast Cancer Patients - Medscape - Sep 15, 2008.












Comments