August 10, 2015
Although the medical device industry is poised for incredible possibilities, it must be prepared to deal with some growing pains along the way.
Lisa Weeks
These are exciting times to be working in the medical technology industry. Scientists are now beginning to create custom human body parts on demand, breathing new life into the concept of personalized medicine. Mobile health (mHealth) applications have the potential to empower patients to take charge of their own healthcare. And medical devices are becoming more interoperable and interconnected, paving the way to a more productive, safer, and less expensive device market.
But as I point out in a more-comprehensive white paper on the subject, the device industry should study both the promise of new technologies like 3-D printing and wireless technology, while keeping track of the risks they embody. 3-D printing, for example, has the potential to disrupt the regulatory status quo while raising questions related to intellectual property protection. And cybersecurity of medical devices is becoming a growing concern as wireless technology advances and becomes more pervasive in them.
Trend 1: 3-D Printing: Made-to-Order Body Parts
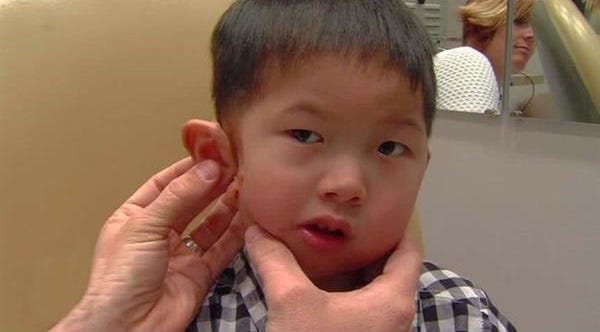
Four-year-old Kai Sherwood was born with an underdeveloped right ear. It's a congenital defect, his adoptive mom believes, that prompted his biological parents to abandon him on the roadside when he was two days old.1 Kai's condition is known as microtia, which means "little ear." Children born with microtia have a small, malformed or absent outer ear (or ears). The deformity is rare, occurring in one out of every 6000 to 12,00 births, but its effects can be physically and psychologically devastating. In Kai's case, it made it virtually impossible for him to find adoptive parents in his native China, where 98% of the country's orphaned and abandoned children are disabled.2 The majority of these children will spend their childhoods, perhaps even their entire lives, living in overcrowded institutions that lack the minimum standards of care. Some will die.
But Kia got lucky--not once, but twice. In 2013, he was adopted by a Utah couple. In March of 2015, he received a prosthetic ear at Salt Lake City's Huntsman Cancer Institute. To make Kai's new right ear, doctors took 3-D scans of Kai's left ear and then used the scans to print a 3-D plaster replica. Using the plaster mold as a guide, doctors sculpted a silicon ear and painted it to match Kai's skin tone. The prosthetic ear, which is virtually indistinguishable from his other ear, was attached with surgical glue. When Kai starts kindergarten in the fall, he won't look or feel any different from the rest of his classmates.
Revolutionizing Patient Care
Heartwarming stories like Kai's are becoming more commonplace as researchers continue to find new and truly mind-blowing ways to apply 3-D printing techniques to the medical device field. In the past, the technology had been used sparingly by device manufacturers, primarily to create products that required only simple materials such as polymers, acrylics or ceramics (dental labs were early adopters).3 Today, the technology, also called additive manufacturing (AM) and direct digital manufacturing (DDM), is being used to fabricate custom-made implants, prosthetics, fixtures and surgical tools on-demand. Some say 3-D printed human bones and organs are not far off. Others argue that the technology will have to improve significantly before we can bioprint a heart, kidney or another complex organ. However, both sides agree that 3-D printers are transforming the way medical care is practiced and delivered worldwide, particularly in emerging and war-torn regions where there is a high demand for (but limited access to) prosthetics and other devices.
The breakthrough technology not only saves lives but also time and money. Three-dimensional printing is much faster than traditional, i.e., subtractive, methods of manufacturing prosthetics and implants. It's cheaper and produces less waste, too. This is a huge benefit to device companies that have low production volumes or produce intricate parts that require frequent modifications. Still, there are drawbacks.
IP and Regulatory Challenges
Today's 3-D printers can copy almost anything, which makes products more vulnerable to intellectual property (IP) theft. The research firm Gartner predicts an annual global loss of at least $100 billion in IP by 2018.4 There are regulatory challenges to confront, too, according to Steven Pollack, the director of FDA's Office of Science and Engineering Laboratories. Although 3-D printing techniques have different technical considerations than standard manufacturing, devices constructed using additive manufacturing techniques are subject to the same regulatory review standards as traditionally manufactured devices.5 The fact that the FDA is addressing 3-D printing at this early stage of its adoption in devices should convince medtech to pay close attention to it in 2015 onwards.
Trend 2: Mobile Health: An App a Day Keeps the Doctor Away
Thanks to the rapid proliferation of smartphones, mobile health--now often simply called mHealth or digital health--has gained significant momentum over the past couple of years, and the trend shows no slowing of slowing down. More than half a billion smartphone users are expected to be using mobile health applications this year. That number is projected to climb to 1.7 billion by 2018. This is pretty significant when you consider that the worldwide smartphone market is projected to grow to more than 2.56 billion by that same year.6 If these projections prove correct, more than half of the world's smartphone owners will be using mHealth tools to manage their health in 2018.
Survey: Doctors Are Ready to Embrace mHealth
Doctors in the U.S. are slowly getting on board the mHealth train. According to a PricewaterhouseCoopers Health Research Institute (HRI) survey, nearly 90 percent of U.S. clinicians think mobile apps will become essential to patient health management over the next five years.7 Seventy-four percent of respondents said they would be willing to use data streamed from a mobile app/device to check for ear infection; 53 percent were in favor of using a mobile app/device to analyze urine, and 20 percent have already prescribed nutrition and weight loss apps.8 Still, U.S. doctors have a long way to go before they catch up with doctors in the U.K. who have been prescribing wellness apps successfully since 2012.
Startups Dominate
The development of mHealth tools like FitBit and their corresponding apps is particularly attractive to start-ups, the current market dominators, because wearables take less time to bring to market than traditional devices. They're also subject to less, or different, regulatory scrutiny. In February of 2015 the FDA issued a final guidance which makes it clear that they consider low-risk general wellness products out of their purview. However, studies indicate that having FDA approval can provide a much-needed competitive advantage in today's highly, some might say overly, saturated app market.
With more than 50,000 free and nearly-free app products available, the competition is stiff. Wellness and other nonregulated apps will enter the market faster and at a lower price point, but they may get lost in the endless stream of new software that floods the App Store daily. Apps that add real value are those that provide diagnostic and treatment capabilities, features that will require regulatory approval. Although these apps will take longer to bring to market, they may offer their developers a competitive advantage. Studies indicate that both consumers and physicians consider FDA approval to be a determining factor when deciding whether to purchase or prescribe an mHealth app.9 Device and software manufacturers should bear this in mind in 2015.
Trend 3: Heightened Cybersecurity Fears
The recent breach of 80 million Anthem insurance subscribers and employees has put data security in the spotlight, again. But many security experts are wondering why the industry didn't learn its lesson last year. After all, inadequacies in health care security are hardly a new revelation. According to the Identity Theft Resource Center, since 2012, the health care industry has suffered more data breaches than any other industry; a whopping 43% of 2014's security breaches targeted health care companies.10 In October alone, the Department of Homeland Security investigated more than 20 suspected cases of cyberthreat in hospital equipment and medical devices, bringing some well-known health care giants under scrutiny.
Had the industry heeded security pudits appeal to increase health IT security spend in 2014, perhaps Anthem could have avoided the mega hack that is predicted to cost them millions, if not billions, of dollars. According to CNET, the company has only $100 million in cyber security insurance, although a spokeperson for Anthem has denied that CNET would have any knowledge of their policy.
"Everyday Devices" are the Most Vulnerable
As medical devices become more interconnected, they also become more vulnerable. Contributing to the risk is the fact that hospitals and other providers do not upgrade their software as often as they should in fear of falling out of FDA compliance. This means that important, frequently used medical devices, such as health and blood pressure monitors, can be manipulated to display incorrect vital signs and cause doctors to provide incorrect medical care. Fortunately, hackers would much rather steal records than reprogram pacemakers; it's far more lucrative. Medical-identity information is worth 10 times more than credit-card information--about $5 to $10 per record--on the black market.11
As other industries, such as banking and retail, continue to beef up IT security, hackers will be looking for alternative, more vulnerable targets. Medtech companies fit the bill. To put consumers' and manufacturers' minds at ease, the FDA and FTC have stepped forward to give the industry some guidelines for ensuring device and data security. The key, they say, is to be proactive.
The FDA's cybersecurity guidance recommends that device makers consider cybersecurity risks as part of the design and development process, not an afterthought. Similarly, the FTC's "Internet of Things (IoT): Privacy and Security in a Connected World" report offers six recommendations that IoT-style devices, which include medical and clinical devices, need to maintain good security. The proliferation of electronic medical records (EMR), networked devices, mHealth applications, and cloud-based technologies has added to the complexity of information management. In this new digitized health economy, balancing convenience, safety and privacy will be an ongoing challenge this year--and for many years to come.
Lisa Weeks, a marketing communications specialist at MasterControl, writes extensively about technology, the life sciences, and other regulated environments. Her two decades of marketing and advertising experience include work with McNeil Pharmaceuticals, SAP AG, SCA Mölnlycke Health Care, Crozer-Keystone Health Systems, and NovaCare Rehabilitation/Select Med.
Sources:
Simonsen, Heather, "3D Printing Gives 4-year-old Boy New Ear," KSL.com, March 27, 2015. (https://www.ksl.com/?sid=33988084)
Vanderlippe, Nathan, "The Tragic Tale of China's Orphanges: 98% of Abandoned Children Have Disabilities," The Globe and Mail, March 21, 2014. (http://www.theglobeandmail.com/news/world/the-tragic-tale-of-chinas-orphanages-98-of-abandoned-children-have-disabilities/article17625887)
Gartner Newsroom. (2014). Gartner Says Uses of 3-D Printing Will Ignite Major Debate on Ethics and Regulation [press release]. Retrieved from http://www.gartner.com/newsroom/id/2658315
Sparrow, Norbert, "An FDA Perspective on the Use of 3D Printing in Medical Applications," Plastics Today, January 5, 2015. (http://www.plasticstoday.com/articles/FDA-perspective-on-use-3D-printing-medical-applications-150105)
Curtis, Sophie, "Quarter of the World Will Be Using Smartphones in 2016." The Telegraph, December 11, 2014. http://www.telegraph.co.uk/technology/mobile-phones/11287659/Quarter-of-the-world-will-be-using-smartphones-in-2016.html
PWC Health Research Institute, "Clinician Survey," 2014.
Ibid
PWC Health Research Institute, "Top Issues Consumer Survey," 2014.
Chickowski, Erika, "Anthem Breach Should Convince Health Care to Double Down on Security." February 6, 2015. http://www.darkreading.com/anthem-breach-should-convince-healthcare-to-double-down-on-security/d/d-id/1319011
Appleby, Julie and Hernandez, Daniela, "Can Hackers Get Into Your Pacemaker?" The Atlantic. November 20, 2014.
Refresh your medical device industry knowledge at MEDevice San Diego, September 1-2, 2015. |
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like


