Results
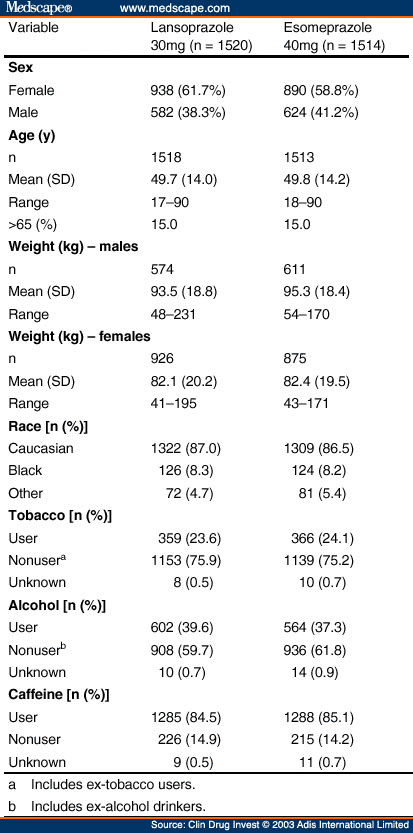
A total of 3034 patients from 360 sites were randomised to the two treatment groups: 1520 to lansoprazole 30mg and 1514 to esomeprazole 40mg. No significant differences in demographic parameters were noted between the treatment groups ( Table II ).
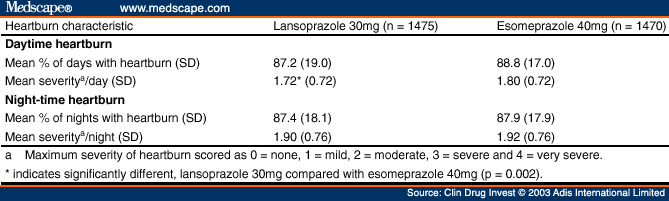
Analyses of the pretreatment heartburn data revealed that at baseline the treatment groups were similar with respect to percentage of days with heartburn, percentage of nights with heartburn, the average severity of night-time heartburn, the percentage of days or nights with heartburn, and the average severity of daytime or night-time heartburn ( Table III ). A statistically significant difference was observed in the average daytime heartburn severity between patients randomised to lansoprazole 30mg compared with those randomised to esomeprazole 40mg (mean severity scores of 1.72 and 1.80, respectively; p = 0.002).
Ninety-seven patients (49 randomised to lansoprazole and 48 to esomeprazole) withdrew from the study prematurely (Figure 1). The primary reasons for premature discontinuation were: 'other' (primarily patient IVRS noncompliance or failure) that occurred in 31 lansoprazole patients and 31 esomeprazole patients, adverse event (lansoprazole, six patients; esomeprazole, seven patients), poor compliance (lansoprazole four, esomeprazole six), lost to follow-up (lansoprazole three, esomeprazole two), personal reasons (lansoprazole four) and symptomatic therapeutic failure (lansoprazole one, esomeprazole two).
Patient disposition flow chart. A total of 3034 patients were randomised: 1520 to lansoprazole 30mg once daily and 1514 to esomeprazole 40mg once daily. A similar number of patients in each treatment group prematurely discontinued from the study; the majority of those for 'other reasons' were due to noncompliance with or failure of the interactive voice response system.
Overall, patient medication compliance was high, with approximately 92% of patients taking >90% of their study medication in each treatment group (lansoprazole 91.7%, esomeprazole 92.7%). Efficacy Analyses There was a statistically significant difference observed between the treatment groups in the average daytime heartburn severity at baseline during the pretreatment period. Subsequently, additional analyses of comparisons between the treatment groups were performed stratifying for daytime and night-time heartburn severity. No statistically significant differences were observed between the two treatment groups in all heartburn relief parameters on day 1 or during the first 3 days, the first week or the 2-week treatment period when the adjustments were made.
Heartburn Relief on Day 1 of Treatment. No statistically significant differences were observed between the lansoprazole 30mg and esomeprazole 40mg groups in heartburn relief parameters on day 1 of treatment ( Table IV ). On the first day of treatment, approximately 45% of patients who received lansoprazole 30mg or esomeprazole 40mg reported being without daytime heartburn. Among those with daytime heartburn, the mean severity decreased by approximately one severity score from baseline during the pretreatment period in both treatment groups. The mean maximum daytime heartburn severity score was approximately 0.8 in both treatment groups. The percentages of patients without night-time heartburn and the average severity of night-time heartburn were similar to the daytime heartburn results.
Heartburn Relief During the First 3 Days of Treatment. During the first 3 days of treatment, no statistically significant differences were observed between the lansoprazole 30mg and esomeprazole 40mg groups in daytime or night-time heartburn prevalence or the severity of daytime or night-time heartburn ( Table IV ). Patients treated with either lansoprazole 30mg or esomeprazole 40mg reported having daytime heartburn on approximately 50% of days during the first 3 days of treatment. A comparable percentage of nights with heartburn were reported by patients in each treatment group during the first 3 days of treatment. The average severities of daytime and night-time heartburn in each treatment group during the first 3 days were slightly lower (between 0.69 and 0.78) than those scores reported after the first day of treatment (range 0.81-0.86).
Heartburn Relief During the First Week of Treatment. Among those treated with lansoprazole, day-time heartburn severity was significantly lower compared with those treated with esomeprazole (0.59 vs 0.63, respectively; p = 0.032). However, no statistically significant difference in daytime heartburn severity during the first week of treatment was observed between the treatment groups after adjusting for daytime heartburn severity at baseline during the pretreatment period. During the first week, the percentages of nights with heart-burn reported by patients in each treatment group were similar (approximately 42%). The severity of night-time heartburn was also similar (lansoprazole 0.66, esomeprazole 0.68).
Heartburn Relief Over the Entire 2-Week Treatment Period. The percentages of days or nights that patients in either treatment group did not experience day-time heartburn or night-time heartburn rose in a linear pattern during the 2-week treatment period (Figure 2 and Figure 3). Of patients who experienced heartburn, the average daytime and night-time heartburn severities decreased in a linear fashion during the 2-week treatment period. Diary data for the entire 2 weeks of treatment showed lower average daytime heartburn severity among those treated with lansoprazole or esomeprazole (average heartburn severities 0.52 and 0.56, respectively) than those scores reported at baseline during the pretreatment period (range 1.72 1.92).
Days without heartburn. During the 2-week treatment period, the percentages of days without daytime heartburn increased in both the lansoprazole 30mg and esomeprazole 40mg treatment groups.
Nights without heartburn. Similar percentages of nights without heartburn were reported in the lansoprazole 30mg and esomeprazole 40mg treatment groups.
The percentages of days with heartburn were similar among those treated with lansoprazole or esomeprazole (36.2% and 38.2%, respectively; p = 0.090), as were the percentages of nights with heartburn (38.3% and 38.6%, respectively; p = 0.606), the average severity of night-time heartburn (0.60 and 0.61, respectively; p = 0.563).
Sustained Resolution of Heartburn. Among those treated with lansoprazole or esomeprazole, the cumulative percentage of patients with sustained heartburn relief was similar on each day of treatment (Figure 4). The percentage of patients who achieved sustained heartburn relief remained virtually stable at approximately 33% in both treatment groups after day 7. Due to the short treatment period, by day 14 less than 50% of patients in each group achieved sustained resolution of heartburn. The 25% quartile of time to sustained resolution of heartburn was 4 days for patients treated with lansoprazole 30mg once daily or esomeprazole 40mg once daily (p = 0.647).
Sustained resolution of heartburn. The cumulative percentage of patients who achieved sustained resolution of heartburn was similar in the lansoprazole 30mg and esomeprazole 40mg treatment groups.
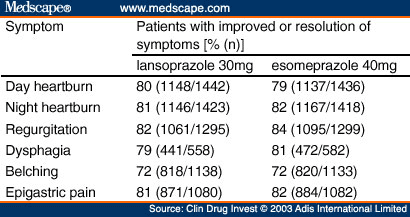
Investigator Symptom Assessment. Relief of GERD symptoms of daytime heart-burn, night-time heartburn, regurgitation, dysphagia, belching and epigastric pain were comparable between the lansoprazole and esomeprazole treatment groups. The percentages of patients with improved or resolution of symptoms from baseline were similar in the lansoprazole and esomeprazole treatment groups at the week 2 visit ( Table V ). Approximately 80% or more of patients receiving lansoprazole or esomeprazole reported having improvement or resolution from baseline in their symptoms of day heartburn, night heartburn, regurgitation, dysphagia and epigastric pain. Improvement or resolution from baseline in belching was experienced in 72% of patients.
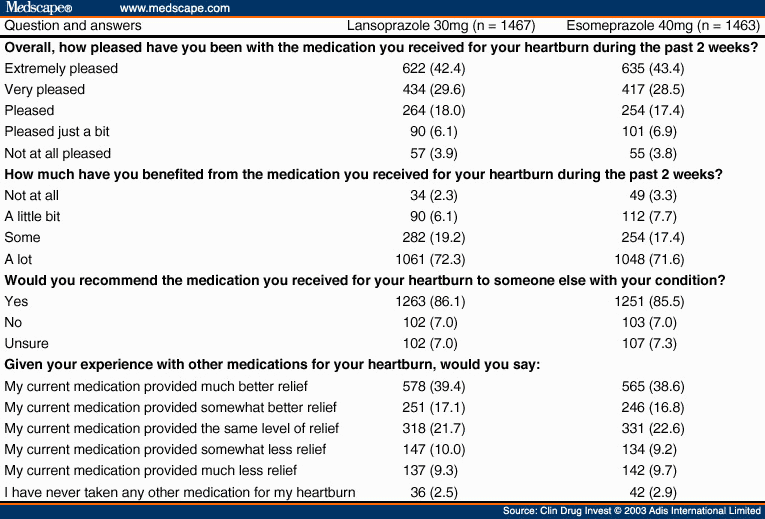
Patient Treatment Satisfaction. At the 2-week visit, 72% of patients in each treatment group reported being 'extremely pleased' or 'very pleased' with their medication ( Table VI ). A similar percentage of patients in the lansoprazole and esomeprazole treatment groups reported having 'benefited a lot' from the medication that they received for their heartburn. In both treatment groups, approximately 86% of patients stated that they would recommend the heartburn medication that they were treated with to someone else with their condition. Greater than 55% of patients treated with lansoprazole 30mg or esomeprazole 40mg once daily reported that the medication provided 'somewhat better' or 'much better' relief compared with other medication(s) that they had taken for their heartburn.
Less than 10% of patients in the lansoprazole 30mg (9.1%, 139/1520) and esomeprazole 40mg (9.8%, 148/1514) treatment groups experienced any treatment-emergent adverse event. Two treat-ment- emergent adverse events occurred in a significantly greater percentage of esomeprazole-treated as compared with lansoprazole-treated patients. Eight patients (0.5%) treated with esomeprazole reported dry mouth compared with one lansoprazole- treated patient (0.1%, p = 0.021). Seven patients (0.5%) treated with esomeprazole reported vomiting compared with one lansoprazole-treated patient (0.1%, p = 0.038).
Less than 6% of patients in either treatment group experienced an adverse event considered possibly, probably or definitely treatment-related (lansoprazole 5.2% [79/1520], esomeprazole 5.7% [86/1514]). The most common (occurring in ≥1% of patients) possibly, probably or definitely treatment- related adverse events were diarrhoea (lansoprazole 1.4% [22/1520], esomeprazole 1.8% [28/1514]), flatulence (lansoprazole 0.7% [11/1520], esomeprazole 1.0% [15/1514]) and abdominal pain (lansoprazole 1.1% [16/1520], esomeprazole 1.3% [19/1514]).
Seven adverse events were considered severe: one episode of diarrhoea (esomeprazole) and one episode each of asthenia, diarrhoea and nausea/ vomiting, dizziness, insomnia and yawning (lansoprazole). Five patients treated with esomeprazole (one accidental injury, four cardiovascular events) and two patients treated with lansoprazole (one back pain, one hypertension) experienced serious adverse events requiring hospitalisation during the study. None of these events were considered related to lansoprazole or esomeprazole treatment.
Eight esomeprazole-treated and six lansoprazole- treated patients prematurely discontinued treatment due to an adverse event. Of these, four patients experienced abdominal pain, dizziness, nausea, vomiting, flatulence and/or increased cough considered possibly due to their esomeprazole treatment and five patients experienced abdominal pain, diarrhoea, flatulence, dizziness, nausea, petechial rash, peripheral oedema, and/or laryngismus considered probably or definitely related to lansoprazole treatment. No clinically significant changes in systolic or diastolic blood pressure, pulse rate, temperature, respiratory rate or bodyweight were noted in either treatment group.
Clin Drug Invest. 2003;23(2) © 2003 Adis Data Information BV
Use of tradenames is for product identification only and does not imply endorsement.
Cite this: Lansoprazole and Esomeprazole in Symptomatic GERD - Medscape - Feb 01, 2003.