Medical device company Medtronic released designs for one of their ventilators to open source for use in the COVID-19 pandemic. This is a laudable action, and there is plenty to glean from the specs (notable is that the planned release is incomplete as of this writing, so more info is on the way). Some initial reactions: medical devices are complicated, requirements specifications are enormous, the bill of materials (BOM) is gigantic, and component sourcing, supply chain, assembly, and testing are just as vital as the design itself.
The pessimist in me says that this design was open sourced for two reasons; to capitalize on an opportunity to get some good press, and to flex in front of the DIY community and convince them that the big boys should be the ones solving the ventilator shortage. The likelihood of anyone actually taking these specs and building it as designed are essentially zero for a variety of reasons, but let’s assume their intent is to give a good starting point for newer changes. The optimist in me says that after what happened to California over the weekend with 170 ventilators arriving broken, it might be nice to have open designs to aid in repair of existing non-functioning ventilators.
The design details released today are for their PB560 model, which was originally launched in 2010 by a company called Covidien, before it merged with Medtronic, so we’re already starting with a device design that’s a decade old. But it’s also a design that has proven itself through widespread use, and this data dump gives us a great look at what actually goes into one of these machines. Let’s take a look.
Requirements Documents
There are a few very long documents in this section, but the overview is that this describes a document for a medical device with a lot of features and a lot of requirements, designed and manufactured in an orderly timeline. Two points that are worth mentioning are that these documents are great for looking at all the technical requirements of a ventilator, and that many pages can be crossed off in the name of expediency.
Electrical Schematics
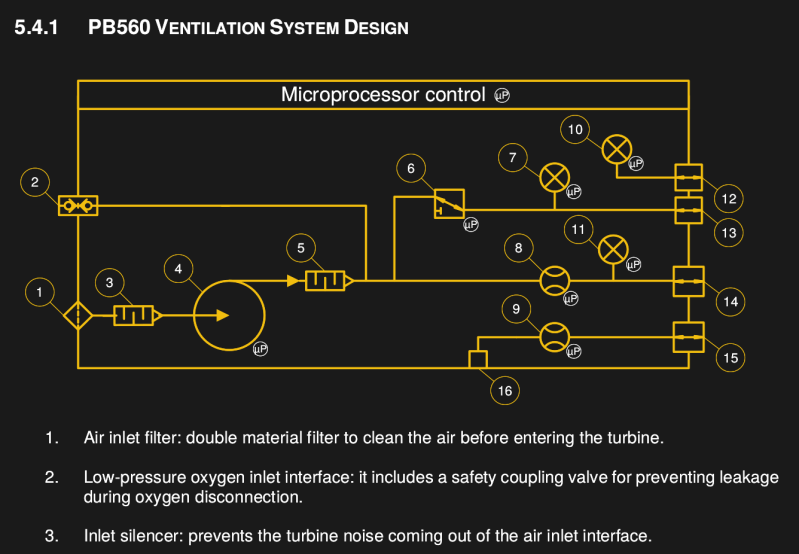
The design functions around an ST10F27? (where ? is either an 8 or a B or a 3, depending on how you squint when looking at the PDF). There’s an ST10F273M that’s currently in active production by ST, so that might be it. There isn’t a full BOM available yet, but looking at some of the components shows that they are still available. There are no less than 4 different PCBs, with a main board, a board for the buzzer, a board for the power pack connection, and a board to control the motor.
Manufacturing Documents
There are lots of pretty pictures inside the manufacturing documents that show how the unit is assembled, with some overview documents that show the details of the assembly workflow. It seems there are some documents missing, though, because there’s nothing about the blower itself.
What’s Missing
Despite it being a dump of 53MB, there’s quite a bit missing if you were trying to build this machine. However, Medtronic did mention in their press release that “…software code and other information will follow shortly.” so there are more details on the way. Here are the things we’d like to see:
- Firmware
- Mechanical design
- PCB layout
- Complete BOM, not just of the electronics but of all the components
- Programming, test, and assembly fixtures
We Need More Medical Devices Published as Open Source
Thank you, Medtronic. This is a great step, and we hope that useful information can be gleaned from this available design, and that others will follow your lead. While it’s not possible to recreate the product with the currently available files, it is a revealing view of the complexity involved in not just ventilators but any medical device.
We hope that this could be used to repair existing units that are not in service. Already, having the service manual and an explanation of the testing process is a huge help in this area. If they were also to release the mechanical design it would technically become possible to fabricate replacement parts to the original specifications if OEM replacements were for some reason unavailable.
However, we suspect that the amount of work that would be required to spin up assembly of this particular product is more than could be accomplished in the amount of time available, and the resources that would have to be mobilized are probably the same resources already working on building medical devices for other designs. The documentation around the release says any products released based on this are only to be used for COVID-19, so if anyone does manage to take this and use it to start production in a timely manner it will be both incredibly helpful, and super impressive.
2020-03-31 Update:
Medtronic delivered on many of the missing documents, and have promised to release the rest soon. This release includes all of the mechanical design files, the electronic design files, BOMs, fixtures, and other components like wiring harnesses. It is yet another indication of the complexity of this product and the challenges that would have to be overcome to source all of the components needed to manufacture it.













everyone is calling bull%%@t on this. a PR stunt.
This is the kind of PR stunt we can do with.
Even if COVID-19 wasn’t ravaging us I’d be happy with this.
Of course it’s a PR move, but it’s also one that’s essentially a unqualified good.
What is missing: the s**t that I need to buy/fabricate!
This is not for you. This is for people who might be able to make one. The idea is that there may be companies out there that produce something vaguely similar. Who knows, maybe some sort of industrial machinery that actually has similar parts. They might be able to actually produce these. Especially with technical assistance from Medtronics and perhaps partnering with some other companies for parts they cant make. And yes, there is also the point of letting people see just how complex these things are. A lot of people with a little knowledge are complaining that the ventilator manufacturers and especially their partners like GM are “dragging their feet” making ventilators.
After looking at those designs, consider what GM has done. Within a few days of partnering with a ventilator manufacturer, they have managed to source ALL of the parts through their global supply chain partnerships, develop a plan to begin production, and send their engineers to learn how ventilators are made. Look at those designs. How practical is it for GM to “Start making ventilators now!” at an abandoned automobile factory (that they no longer even own) which was fitted out to stamp massive steel components like car bodies, paint them and then assemble large heavy assemblies like engines and axles into them and then finish them off by fitting the interior components and sheet metal components onto them. Its like apples and oranges. So now that you have read those, it should be clear that those demands were nothing short of absurd, and ill informed. That in itself is not a bad reason for them to have released those plans. It will help give the public realistic expectations of what can be done.
I think we have all been in that situation where someone in charge (or who thinks their in charge) tells you to do something that just is not possible.
Your boss says “I want these new PCs prepped and on users desks by Friday” and your trying to tell your him “It time to PC from the network and another 45 minutes to install it and get the final configuration done, and that cloning time goes to about 2 hours if we have a dozen of them cloning at once which is the maximum that we can do, and there are 600PCs to be done so if they are going 24 hours a day then we can do 144 a day. So it will take 4 days just to get them cloned. And its already Tuesday. There might be some things I can do to speed it up. Of course while Im doing that, Im not cloning or installing PCs. But that’s not the real problem as installations can happen while other systems are cloning. So the installations themselves are the bottleneck. It will take about 19 man days to install all 600 systems. So if you want it done in 4 I’m going to need at least 14 people to help me and we will have to work around the clock. That’s assuming that everything goes perfect which in the real world it never does”
And your boss says “There is no one else available to help you, I don’t want to hear excuses, just get it done!”
The reality is, no matter how much he threatens you, no matter how much he yells at you, it simply will not get done by Friday.
Exactly. There was never an intention that this should be like a Heathkit. And realistically, there’s no non-trivial open source hardware project that is as well documented and buildable as a Heathkit. Just because someone publishes Gerbers and a BoM doesn’t make it buildable and testable.
I think Medtronic did the world a service – There’s a whole long way between diagram of ventilator on Wikipedia and an actual producible design. It’s true that one might design something simpler or different than the Medtronic unit (technology has advanced in the last 10 years), but certain aspects are going to the same, or need some similar attention to detail.
-1 What is this “everyone”? Doesn’t include me!
Here’s one of the reports about it.
EEVBlog on Medtronic Ventilators: https://www.youtube.com/watch?v=1wrin8FYtEo
Seems like a PR win: instead of being labelled as evil money-grabbing big pharma nasties who put profit over lives and don’t want “this one secret trick to building your own ventilator with a balloon and two paper clips that casinos are trying to stop you knowing, find out how local housewife makes $50,000 a month” to be known, they’re publishing docs for an old (= simpler) model, and we can all see ourselves that they’re not being evil, these things are actually complex. And the best thing we can do is actually to stay at home and wash our hands.
When they say 170 respirators were “broken” did that mean entirely non-functional or just that filters, bellows, or other wear susceptible, normal wear and tear, or expired parts had to be replaced (and perhaps for recertification). Other respirators in California storage had to have some sections rebuilt after being unused after 10 or more years.
I am curious also. I couldn’t find any details of the fault on line. Maybe flat PCB battery’s
It can probably run the whole gamut, elastomeric parts gone too hard, cracking and crumbling, mechanical stiction of shafts and wipers, solidified lubricants, leaky electrolytics, blowing tantalums, settings backup batteries leaking, other types of NVRAM scrambled from 10 years of cosmic ray hits and no runtime single bit error corrections, slight contact oxidations in any connections, tin whiskers growing unchecked, and the old favorite, you didn’t know whether it worked or not so it decided to dick around with you.
And look who got the job ($$$s) to restore those “broken” respirators in CA. Experts in the field of … what, wait, they aren’t ?
The true story seems a little bit different “The State had a stockpile of ventilators sitting idle in a warehouse in critical need of refurbishment. A service provider for ventilators told the Governor it would take a month to do the refurbishment.”
These ventilators were known to be non functional, it is not as spectacular as what the governor tweeted.
But still this is an amazing work fixing it in days instead of a month:
https://www.bloomenergy.com/blog/were-stepping-aid-covid-19-battle-we-need-your-help
From what I have read, ventilators have to be professionally maintained in a working condition. They are not made for long term storage. So if they sit on a shelf for 10 years, they probably wont work. I suspect its an issue of things like rubber components and such. Imagine a rubber seal sits on a bore for 10 years. It gets no lubrication and is slowly oxidized by air and is permanently deformed from constant applied mechanical pressure between the parts its sealing. When you fire the unit up that seal will fail immediately.
It seems to me that there is a need for a special “Stockpile” ventilator. Imagine a device made from materials that do not degrade. Metals would be preferred over plastic for critical components etc. Electronics would be made avoiding components like some kinds of capacitors that have a shelf life. Critical electronic components would be carefully chose to be readily available for a long period of time. A emphasis on simplicity would be critical. For example, ventilators today have many features that are simply not necessary in such a machine. Just keep it as simple as possible to provide the core functionality necessary. Such a machine would probably not be stored fully assembled. Imagine a machine where all the pistons and such are sitting in a blow formed plastic tray. The device would be designed so that these parts could be installed without tools by the hospital staff following a detailed instruction sheet that was affixed to the inside of the shipping case.(thumbscrews would have massive threads and be designed to be resistant to cross threading and incorrect installation. In other words, you would not put a cover for the piston with four 3″” thumbscrews in an aluminum bore. You would have a stainless housing and thumbscrews with a massive 8mm thread such that its not possible to strip it out by hand. Rather than having a torque specification you might have screws with springs that have a shoulder that bottoms out ,creating appropriate holding force for a spring (captive on the screw) in a way that is repeatable without tools or skill.
You would open a sealed bag of O-rings and seals, install them as per the instructions, place those parts in the machine and affix the covers with thumbscrews. Then turn the machine on and run self diagnostics. Electronics would be designed to have a long shelf life. The parts would all be designed to swap out without tools so that they could be replaced easily and self diagnostics rather than saying stupid things like “X-0 Pressure fault. Call service” or other meaningless message would say things like “Electronics Module failure. Replace the electronics module and re-run diagnostics” In other words, it would be designed to tell you what was wrong and exactly what part to replace to fix it. When such a machine was returned to the stockpile it would be tested, then disassembled, the rubber seals discarded, a new package of seals placed in the box and then a tag placed on the case (so that the tag would have to be broken to open the case) verifying that it was prepped for long term storage ,and then the device would be returned to the stock pile. The design specifications would include the maximum amount of time such a machine was expected to remain mothballed before it had to be removed from storage, setup, tested and re-mothballed.
This is interesting! I have been keeping a list of your Ventilator articles on my ponned Quora answer and have been updating it daily.
I work in the medical devices industry, and of course there are FDA implications to manufacturing something like this. But we are going through something nobody has seen in the West for generations, and that requires us to make adjustments at all levels.
It’s cool, but not open-source. The license isn’t compliant with the requirements. The worst offense, it reverts to proprietary in 2024.
It is open source… but not under a recognized license, there are plenty of open source projects that are incompatible with many open source ideals at large. The limited term doesn’t preclude it from being open source.
Since there is no transfer of property you might claim it is an open proprietary license but many fully recognized oepn source licenses have the contributors transfer ownership of to an entity etc…
When the term “Open Source” was coined, the group that was working on it also wrote guidelines that define Open Source. The OSI is the group that now handles approving new licenses. The term for a license like this one is “source available”. For more information: https://en.m.wikipedia.org/wiki/The_Open_Source_Definition
It has too many restrictions to qualify as “open source”.
We should petition them to release it under the CERN Open Hardware License:
https://www.ohwr.org/project/cernohl/wikis/uploads/002d0b7d5066e6b3829168730237bddb/cern_ohl_s_v2.txt
And worse yet- Even if you could copy it exactly, the FDA won’t allow you to use it in a hospital without it’s own FDA approval, even if it was built in an FDA approved facility. So, unless you have said facility already, and know people in high places, “open source” or “hack your own” ventilator projects like we see here on Hackaday make no difference, other than to cause discussion.
I am gonna start the “Bring Your Own Ventilator (BYOV)” movement.
That’s true in the US, and quite a few developed countries, but not everywhere.
maybe it’s just a counter fire to this article.
https://www.nytimes.com/2020/03/29/business/coronavirus-us-ventilator-shortage.html
Another case of things that are publicly funded not being in the public realm. That allowed mega corp medical company to undermine the low cost offering that was being funded to fill the emergency stockpile.
Is this the famous respirator that was designed for FEMA to be mass produced at low cost?
https://www.nytimes.com/2020/03/29/business/coronavirus-us-ventilator-shortage.html
I seriously doubt it. I’ve been roped into a ventilator project and considering the circumstances and time frames we are keeping it uber simple. That said, this thing is so totally the opposite it is crazy complicated and there is no way on earth you could make this cheaply even at the medical device worlds idea of ‘cheap’. Just too much stuff in this thing and way too much time in the assembly.
What disturbs me most is that the complexity of this design will put others off who might have otherwise produced something sufficient for the job and a real life saver. Publishing this might actually cost lives in that way. If they had published an older design, lower tech and simpler it would not have cost them sales because medical world would happily discard them after the pandemic and they may be available sooner and from more places.
This is a cynical, ill conceived stunt which IMHO should be shunned. For reasons look at what others have found: the 2024 expiration of the license and the fact that the license is incorrectly stated as open source all say to me this is not born of altruism at all but something rather darker.
The complexity behind it might have a real medical purpose.
Maybe the simple device you envision covers 80% of the use cases. But then you add something more to cover 90%, and something more to cover 95% etc.
Then there is reliability, self diagnosis and the actual responsibility that the device has to actually work.
It’s very likely in the end doctors would prefer the type of device that covers 99% of use cases and is reliable even if more expensive.
Just check the 67 page document with the requirements. I bet there are some you did not think of. (github link somewhere in the comments)
Exactly – The plumbing seems pretty simple and straightforward – valves, a pump, fittings, filters, and sensors. The microprocessor might not be what one would choose today, but it does what’s needed hardware wise.
I think the hard thing on these devices is the Failure Modes Effects Analysis (FMEA) and the code/hardware to do the monitoring. Some of it probably should be done by separate circuitry (if the microcontroller jumps into the weeds, does the patient die, unannounced?) There’s a lot of stuff about self monitoring for faults, the connection to the nurse call loop, all the stuff needed to let it run unattended. If one were willing to have a person there the entire time to do the monitoring, then a conventional bag respirator might work, or one of those “machine squeezing the bag” contraptions (to alleviate the muscle use of the bag squeezing person) But tying up 4 people (4 shifts needed to cover 24/7) per patient is a) really expensive and b) probably impractical – it’s not like we have a lot of trained bag operators around.
Thanks Electrobob, I have designed plenty of devices in a career spanning some decades so I am aware of the points you make and in particular with medical devices as well, but are you aware that in Aus anyway NSW Health has distributed an old commercial ventilator design and is seeking it to be made ASAP because as of the 3rd of April (yes, this week) the ventilators in ICU right across the state will be at full capacity. Given that if the virus affects the lungs then without a respirator you are not looking good for football on Saturday. Any Saturday till the end of Saturdays. So NSW Health has totally relaxed the regulations to make it easy to get this gear into an ICU in an effort to stop a very large number of people dying in the next few months.
They really don’t care if the ventilators are a bit less than a rolls royce if they mean people live.
I probably should have explained all that earlier but now I hope you can see where I was coming from.
But on your point; complexity does not equal reliability, and Jim, to implement safety critical functions in software is usually avoided like it is pure acid. Safety functions and risk mitigation features are in hardware or they don’t exist even if they do. To get that kind of software function through the FDA is a world of woe into which very few but the most intrepid tread.
Stephen, could you provide a link about this please?
(I see your point now…text makes it harder to get the message right sometimes. )
Indeed, in this kind of situation, we should take whatever we get. Something that does the job 70% of the time is a lot better than nothing that does the job 0% of the time.
What i was trying to say is that all the complexity has a meaning that was fit for the purpose of a well designed, reliable and general purpose ventilator, which is what doctors would normally want and that is what the company designed. But in these times of crisis, anything better than nothing can work.
It very well could be (it matches the description given in that article) in which case the irony is that US citizens are the ones who paid for its development in the first place.
Yes, this is the what the failed attempt left behind by Newport. Covidien bought Newport in 2012 and Medtronic bought Covidien in 2015. Essentially, Newport failed miserably at the project and the competitiveness over a potential low cost unit invading the market caused a flurry of company buy-outs and mergers. Health care equipment manufacturers are interested in profits, not your health care.
https://www.motherjones.com/kevin-drum/2020/03/nyt-next-gen-ventilator-project-failed-because-it-killed-profits/
> it might be nice to have open designs to aid in repair of existing non-functioning ventilators.
A service manual and calibration manual would be more useful than trying to learn and figure what went wrong in the machine from looking at the source code. It has all the information of taking things apart, some repair decision tree and parts/assembly code for repair parts.
No one would try to fix a car from the CAD drawing or the firmware. etc. You can fix the bugs in the design or modify the design. Once again barking up the wrong tree.
I downloaded the package earlier, and the service manual is part of it.
It is nice of them to do so.
How often you get that in the average open source projects? Most of the them you get yelled at or told to look at the source code for documentation. Documentation is one of the worst part in a open source because it isn’t fun so no one want to update the document to match the releases. SDCC is one of the exception as they update the documentation as part of every release.
This is Hackaday – of *course* someone’s gonna try to fix a car from the CAD drawing!
Can the FDA relax current class2 purchase restrictions on these devices. In the US, it looks like I can’t buy a new, used or broken ventilator to use, repair or improve. ? If anyone knows a legal way to do this please share.. I understand the wish to protect the general public, perhaps they can at least relax for personal use not for professional/commercial use ?
Anyway, sounds like a great source of useful information and learning about what needs to go into a real medical device. Did they also release/indemnify for use of their relevant patents?
In the whole scheme of things, it would be an utterly illogical thing to do at this time. In the balance, maybe a dozen interested hackers getting one to reverse engineer, which is on the face of it losing a dozen machines, each machine having the potential to save 2 lives a month at least for the next 3 months. So yeah, lets kill 72 people to give those guys a chance to come up with something that might be too late anyway, if they do much at all. Then firing all that up in the air anyway like it was the counterweight on a trebuchet is the tens, nay, hundreds of thousands of people who would suddenly bid the price of a large well equipped car to make sure they have their own respirator or one for grandma, sickly kid, etc etc. So no, it would not be a good idea to do the TP/Sanitiser/mask thing all over again with respirators.
You are spot on. In addition — is this the machine the hospitals are crying for? I have worked designing POC resuscitation devices such as this since the early 90’s for two different companies — and yes — they are not giving you a whole lot here. Before you can supply a device to the medical industry you have to have the specification for what they really need and want. It would be great if they would release the Functional Requirements document which would be key to understanding the design intent along with production drawings. What I have seen so far out in the Maker communities are creative ideas for pumping air into people. None of them seem to be something that a nurse can be confident to walk away from the patient’s side; which is what will need to happen in an overwhelming situation. The toy like look of the unit belighs its sophistication and ability to interface with other systems as indicated in the user’s manual. Good Call —
Spent a while trying to work out why PoC need a different type of resuscitator from white people until I realised that probably wasn’t what your POC stood for. Congratulations world, we’ve run out of TLAs.
Thanks Dan — didn’t realize community not familiar with the acronym “POC” — it stands for Proof of Concept. I think we are gravitating toward Greek, where word usage and meaning are contextual. I think we are running out of letters to form acronyms!
If it is actually Open Source, perhaps somebody who is willing to sign their agreement would re-post the documents somewhere that the general public can download it.
That’s really where the rubber meets the road.
Dave (eevblog) already had debunked this bogus stunt by Medidiocy (clever name, huh?).
https://www.youtube.com/watch?v=1wrin8FYtEo
At a former employer, we made stuff that went into their ventilators. And my experience with them has led me to my personal opinion that that Pitney Bowes/Covidien/Medtronix is/are yet another evil company.
Hackaday writers called it open source. Medtronic does not. Don’t blame them for the mislabeling. They only say that they are sharing their designs.
Frankly, knowing how a lot of medical device companies are, I have to give them a lot of credit for doing this. No matter what license terms they put on this, the reality is after this crisis the market will be flooded with leftover respirators and now freely available, fully tested designs, and they’re shooting themselves in the foot a bit to help the world.
If you’ve even read through the files, it includes rather critical documents, like a PRD, and manufacturing/assembly notes that actually allow other manufacturing firms to understand what the critical requirements of a respirator really are. If you’ve ever put anything serious in production, in volume, you’d know these may very well be worth as much as the design schematics. They’re also notably missing from most open source projects, and really where most of the time spent bringing up an open source design ends up coming from.
Despite their license, they are giving away way more than any company probably ever has under GPL. If you’ve ever seen hat kind of nonsensically useless junk most router manufacturers ‘release,’ you’d probably understand this. What they’re doing looks like the equivalent of not only releasing source code, but all the software developer design notes, toolchains, software testbenches and high level design documents.
Perhaps it doesn’t seem useful to the average hacker, but to anyone who owns spare manufacturing capcity and wants to help – this very meaningfully helps them start up manufacturing lines in places like car factories. There are still potentially critical mechanical design files still missing, but hopefully those will come out soon.
Medtronic’s CEO called it “open sourced” on Twitter: https://twitter.com/MedtronicCEO/status/1244600905858797568?s=20
You make some good points, but while the “this isn’t open source” comments might be pedantic, they’re not unwarranted.
Well, there are huge disputes in the open source community about what constitutes open source. I think what some people are missing here is that there’s a lot beyond “source code” that is needed to produce a working product – how many times has someone downloaded open source software, contemplated modifying it, and said “well, I have no clue how that works, and it’s not worth the months to figure it out”, or gosh, even getting it to compile (you have the source, but don’t have info on which libraries of which versions, etc.).
What Medtronic has released is normally kept as proprietary trade secret – So, from their standpoint, it’s pretty darn “open” – for a manufacturer, the step by step assembly processes, the requirements documents, the regulatory certification paperwork – that’s their competitive edge. Anyone can buy the completed unit and reverse engineer it to figure out the BoM, schematic, and chassis – it’s the “how do you build and test it” that’s the secret sauce, which is what Medtronic has released.
Having a detailed BoM probably isn’t useful – for one thing it would be house part numbers – you’d also need the index that converts “stock number 1234” into an actual purchaseable part – and since this design is 10 years old, I’d bet that if you had supplier part numbers, a lot of them aren’t available any more.
I think to a certain extent, this release might have been prompted by the folks at Medtronic wanting to illustrate that “hey, a *medical device* isn’t something you cobble up from RPi and some spare servos you had in the garage” – there’s a lot of thought and regulatory process that makes sure it’s “safe”. Everyone can and does argue about the burden of regulation and approvals, and can typically cite specific issues, but the fact remains that most of those requirements and regulations exist because “something bad happened”.
I work designing and building spaceflight hardware, which is similar in it’s “criticality” and we have hundreds of “flight project practices” and “design principles” and each and every one of them represents a “lesson learned”, often the hard way.
So, while this may not meet some rms or esr standard of “open source” nirvana, it is a HUGE improvement over what was available before. And practically speaking, Medtronic would have a very hard time succeeding in suing someone over use of the information (as long as it’s not an exact copy). There’s enough copies floating around now, that one can stand up and swear you didn’t execute Medtronic’s agreement. So it’s not “trade secret” – you might have to worry about patents (maybe.. it’s 10 years old, patents have a 20 year life). And if you’re really designing your own, Medtronic’s documents are an excellent example of what you need to do – you’ll need the same kind of documents covering the same kind of material.
“there are huge disputes in the open source community about what constitutes open source. ”
Not really, it’s mainly people outside the open source community who don’t understand. The CEO obviously doesn’t have a clue, nor do their legal people understand what a “permissive license” is.
The license is restricted to 2024 or the end of the WHO Public Emergency, among others things.
The license is nowhere near open source, nor permissive, despite what they claim.
Add the fact the design is based on an obsolete CPU, no one making these will get approved, it’s a massive waste of time and a pubilcity stunt.
Given “What’s Missing,” how is what’s provided significantly better than being given a brick and told to turn it into a ventilator?
More like hitting your head against a brick wall
Unfortunately, the bulk of the negative comments are spot on. Also, yes, this is a remnant of the failed government “low cost ventilator” project from years ago and several articles are already floating the internet stating as such. Medtronic gave up on the project due to cost because they backed themselves into a corner on the design. In other words, they were too closed minded to new methods and tried to create a $3k unit from a $10k design.
Sad that Medtronic is gaining “good PR” for a package that one could replicate by just buying a working ventilator and doing a proper teardown report. However, I will say that much of the theory of operation type data included is worthwhile to someone doing research for a true low cost unit.
Well, I guess a chunk of the cost is using an obsolete CPU which costs $40!
What a joke. PR stunt!!!
Please, just shut up. From the looks of the make up, it is clearly not a PR stunt. It has the look of “we need to do something, we can do, we must do, it does not need to look clean like a company presentation, but understandable”.
I applaud this move!
To set aside commerical interest for some time, common sense, and responsibility to act as a unit – and not just like marauding self centric preppers – in these times to protect society is something I prefer.
Let’s work together – canned heat:
https://www.youtube.com/watch?v=Hom0fYd5uX4
The files are behind a registration wall. I’ve put a copy on GitHub:
https://github.com/abetusk/Medtronic-PB560-Ventilator-System
For everyone unfamiliar with electronics manufacturing, functional safety, and medical devices. Please take a look at the designs and the files they did give us. Take it as data points, try to figure out what makes this thing functional and reliable.
It’s extremely hard to do this right. Every component has purposely chosen. You can’t just swap a 10k pull up for another value or random manufacturer. Not unless you want to redo huge parts of the validation. There are no corners you can cut.
I write this replay as most complaints and ‘solutions’ are offered by people who are not in the industry. I understand most of you can build very cool devices using the tools available. This is not your game. Do offer your time, materials, machines, and energy to your local hospital. Talk with them and ask what they need. Ventilators build out of 3D printer parts is not it.
“You can’t just swap a 10k pull up for another value or random manufacturer.” If what you say is true, than this is worthless without a BOM with manufacture’s part numbers…
They’ve said what they need from us:
– stay at home
– wash your hands
– don’t call 999 or your GP unless it’s a real emergency, no more of this “my kid has a bruise, we’ve got free healthcare so I’m going to take him to A&E to get him checked out” crap
I think this one, as many others, is part of the reason for the new MDR. If you look numbers up. In the last ten years around 600 Class III devices had to be called back. Who’s scared of Corona… if we have enough negligent doctors and medical device engineers.
“medical devices are complicated, requirements specifications are enormous, the bill of materials (BOM) is gigantic, and component sourcing, supply chain, assembly, and testing are just as vital as the design itself.”
Well yes. This device might be a particularly poor representation, but medical devices like a ventilator ARE complicated due to the demands put on them, hence the enormous requirements specs. Those demands and requirements also make that sourcing, supply chain assembly and testing are just as complicated. This is all with good reason. All those specs and requirements are almost literally the result of the deaths of those that didn’t make it in the past.
I have a sneaking suspicion that what Medtronic is really hoping for by making the design proprietary again after 2024, that someone takes this design, runs with it, simplifies it to the point where it IS possible to produce it cheaply. Only to see their design based on something proprietary and Medtronic then coming to them with an offer they can’t refuse. Medtronic then has a cheap ventilator design for basically 0 engineering effort.
However, as stated, to begin with this ventilator design is a particularly poor example to begin with. Even Medtronic itself probably has better designs available and in production. I know other manufacturers are working on the “we need to make way more right now” design simplification on their own products and supply chains are getting set up to increase the production line capacity of known good and proven models.
While I understand the push for people to create their own Rube Goldberg/Heath Robinson devices, I strongly doubt it’s a good idea. I’d much rather see the government going to manufacturers and their suppliers and saying: “How many engineers and workers do you need extra to speed up your redesign or supply your parts faster? What do you need from which of your supplier? Here’s the money to hire engineers from engineering firms with the required knowledge, here’s money to hire more temp workers” There’s plenty of people out of a job, there’s plenty of engineering brainpower available. Plenty of countries are basically in emergency law/wartime mindset. So use this. Switch manufacturing resources! Very little of that is happening in a coordinated way.
Even a simple website where hospitals can put up what PPE and equipment they need and manufacturers can show what they could produce or what stuff they need to produce their products could go a long way to getting things up and running. Everyone is fighting one another right now and everyone is tumbling over one another to scream: “Hey look at me, I’m doing something useful” the loudest.
It’s hard to see how a design, even based on the docs, but without having signed the agreement, would create a problem. It’s not trade secret (there are copies available without executing the agreement). The docs are copyrighted, but anyone can make a new drawing or doc (and would need to for a revised design), and copyright only protects the specific expression. There might be patents (one might go hunting) – However, if the original development were done under federal contract, those patents might actually be assigned to the government (or might not – I’ve seen both scenarios)
But once in the wild like this, claims of “proprietary” are going to be really tough to pursue.
I saw one open source ventilator project from profesionals. They are not private company. They are scientists in the field of artificial ventilation from Czech Technical University in Prague. Check the webpage https://www.corovent.com. It is designed for these days of crisis. Common parts for simple manufacturing. But it will be certified and tested.
A friend of mine called my attention that no source can be found… anyone with a link?
If you ask the medics what they want, as well as the basic mode this Czech machine would deliver, they want patient triggered breaths, CPAP mode and in the case of India, a mask ventilation mode as well, i.e. a complex fully featured ICU ventilator. Also it should be low cost with minimum supply chain issues and capable of being built in huge numbers quickly. These requirements completely conflict with each other. This Czech group in my opinion are much closer to reality, a minimalist industrial type of design that does one thing well i.e. provides the correct ventilation requirements of a sick CoVid patient. If (a big if) they survive that phase and require more advanced weaning modes, transfer them to a better machine. Even if they build their machine to meet the UK MHRA requirements, the MHRA will not at this time test it as they are overloaded. Therefore it will not be used in the UK even if the design proves to be excellent and easy to manufacture.
DID anyone mentioned that open source is NOT capable of delivering this kind of quality to save lives????
Would you hook up your mum to open source ventilator??
You seem to be equating open source with low quality junk. I don’t believe that is always true.
The patent system is supposed to be a little like delayed open source, you disclose how your product works in exchange for a limited period of exclusivity, after which others my copy.
Yes, if it is a sensible design, built by competent people, all regular machines are in use and the alternative is for her to be allowed to die or to put it more politely, Triaged into the group less likely to benefit from ventilation. If a better machine became available and she was doing OK she could then be transferred onto that. One way to never run out of ventilators and ensure ICU surge capacity is never exceeded is to adjust triage guidelines to suit the demand.
“Sensible design” and “competent people” are vague terms in a highly regulated industry like medical devices. Perhaps you would like to be more specific?
While this is a PR stunt and not a true open source license, it does provide several points of useful information that most people who have never been in the industry did not know. 1st, automated ventilators are freaking complicated and as others have mentioned there is probably the equivilent of a whole library of just FMEA documents and testing so that if the ventilator does fail it results in a timely alarm not a dead patient or worse actively harming the patient. 2nd, these appear to be fairly robust, and definitly “over engineered” devices which would also explain the price tags.
Also I have seen several articles talking about the standard production rates for these prior to this pandemic as being less than ten units per day. To go from sub 4000 units made per year to planning on making 50,000+ units by July is insane. that is between a 2500% to 5000% increase depending on if you calculate the manufacture start day as begining of the year or begining of April.
Finally, these companies are going to be looking for a handout or something afterwards because there is going to be a massive surplus of ventilators after this is all said and done and the smart companies should be preparing to shift themselves to a repair/referbishment role to keep the lights on.
It is fun to see that they used different CAD tools for different boards in the system (I smell kicad and maybe OrCad/Allegro)
It is not that unusual. The different divisions (from acquisitions) of my old company uses different CAD tools and have different design priority and process flow. The test engineering group in my other company uses different CAD and process flow because they need to get things done. Since they don’t ship products, they don’t bother/follow the same strict process flow.
“it might be nice to have open designs to aid in repair of existing non-functioning ventilators.”
Good luck with that! The Right to Repair bill has largely failed, you’re gonna void the warranty fixing them yourself or getting an unauthorized repair person to do it!
Hmmm, a ventilator designed 10yr ago by a company called COVIDien, now being used to fight COVID-19. What a strange coincidence
Very
The company name stems from the Latin words: “Co” which stands for together and “vi” for life.
This is not open source. The software license is much more restrictive than other real open source licenses. Medtronic is not your friend https://pluralistic.net/2020/03/30/medtronic-stole-your-ventilator/#market-oxygen
Boycott Medtronics:
“13 years ago, the US Dept of HHS awarded a contract to design low-cost, reliable ventilators to Newport Medical Instrument of Costa Mesa, CA. The ventilators would cost $10K for competing ventilators, but under Robert Bork’s antitrust theory, there was a simple solution.
In 2012, Covidien, a giant in the field, simply plunked down $100m (chump change, given its revenues of $12b that year) and bought Newport.
Then they killed the ventilator project.”
I’m having a Dilbert moment here!
As with everything else in life, it’s more complicated than saying they killed the project. The project was initiated by Newport Medical Instruments, a small outfit. The vent never made the price point in the contract. No company will produce products at a loss. How much profit they make is up to them.
The problem here is that a public body contracting a private company to design and build a product at a specific price is high risk. That’s why we have a market rather than a government controlled central economy. Much better for a public body or university to develop a product and make the design public. Which is what is happening here with the release of the Medtronic design and manufacturing process documents.
Then anybody can build it anywhere and drive costs down. Be interesting to see how it works out!
I just download the files after registration on their Web Site. Nothing was open sourced. just a formal and general schematics with no firmware software’s for all the device parts. it is just manuals and requirements docs and no more !!!. It is very strange that a well known company like it makes this disappoint fakes
IANAL, but looking at the license, it seems to me that they would have an argument to sue you if you looked at these documents and then built a ventilator that you sold past 2024. My guess is that this is a way to ensure that Tesla and GM and all of these other companies that are pivoting to make ventilators get back out of the market once this crisis is over. That might also explain why they make you leave your name and affiliation before you can download the files.
Still, having schematics and service manuals and the like, none of which we had last week, can’t be bad.
Thank you, I scrolled through the comments for this, somebody had to do this :D.
It should be noted that the NYT is reporting that Covidien/Medtronic basically killed production of a cheap stockpilable ventilator within the last ten years, possibly to prevent its competing with their more expensive and elaborate ones. I wonder if this design is the one from the company Covidien bought that was eventually killed. So there’s that. This could just be a PR stunt to make tiny amends for that move that’s now probably killing people, something predicted more than 10 years ago.
https://www.nytimes.com/2020/03/29/business/coronavirus-us-ventilator-shortage.html?searchResultPosition=1
A spanish team of engineers and physicians has developed a ventilator from zero with full open source compliance. Animal tested. Waiting for full project disclosure in a few hours, supposedly only legal matters pending. Stay tuned on twitter.com/ReesistenciaT
https://www.instagram.com/p/B-U8h72jBW2/?utm_source=ig_embed&utm_campaign=embed_video_watch_again
That thing is tiny… I thought ventilators were huge affairs. This thing is smaller than my CPAP machine (which is a bit like a ventilator but much less advanced – it doesn’t actually breathe for you – and definitely not pure oxygen-safe).
I’ve tried to analyze the ventilator system, from a machanical design point of view. AND WHAT A DISAPOINTMENT. The pressure source and the valve arrangements, two of the most important things in a ventilator, are just a huge blackbox in the CAD files.
Hi, do you find drawing of turbine?
Are you referring to this for “pressure source” ?
What do you mean by “valve arrangements” ?
For open source design see this https://www.europeanscientist.com/en/public-health/an-open-source-respirator-for-40-euros-from-a-3d-printer/
WHY Hackaday doesn’t cover MIT’s open source ventilator??? A LOT easier!
https://e-vent.mit.edu
SHAME ON YOU hackaday!
You must have missed it.
https://hackaday.com/2020/03/23/mit-ventilator-designed-with-common-manual-resuscitator-submitted-for-fda-testing/
The advantage to this approach is that the BVM is completely disposable so not much cleanup. Cost for BVMs are around $8 to 12 in bulk. Looks like the lab is well equipped to be able to validate functional requirements of the device. They do need to work on the form factor – the complete works are a bit bulky for a crowded ICU environment.
Medtronic have now added two more large zip files with more documents. I haven’t looked yet but the descriptions suggest there is much more information now.
Hi,
I downloaded all files but I don’t find drawing files of turbine.
This is only a empty box but there are no drawings of the turbine or the used brushless motor.
I think that all is a publicity stunt.
BOM is pretty useless…all internal part numbers. Was keen on finding some valve and flow sensor part numbers…
Just finished going through all the files, hoping to put a flow chart together to build.
ROADBLOCK!!!!!!!!!
The most important part, the turbine, including motor and impeller to deliver the “250 lpm into a pressure of 24 hPa”
is UNKOWN.
I just discovered this TURBINE / BLOWER is not designed or supplied by Medtronic, but by a company called airFan.
Also as surprising, airFan has removed the PDF document for this item, one MFA0114.
Came across a website, that Tesla is making a Medtronic Ventilator as well……..
I am hoping that others with a solution to the TURBINE issue, will reply.
Most likely the motor and fan assy was not off-the-shelf but spec’d specifically (bespoke) for the vent device. Without the spec your back to square one developing your own. You could get a test lung from Michigan Instruments or Siemens so you could test different outputs from motor and turbine combinations and go from there.
I am hoping that anyone here have solution or suggestion for this turbine/blower.
If you have, kindly support us for any alternative supplier, because Airfan is over-capacity now
Save yourself the time, by designing and building your own. The good thing is the driver design is included, you just need to get the motor and impeller design tested.
The Turbine is a small issue compared to the Display problem:
The PB560 is built upon an outsourced LCD driver.
You could get the LCD, but the driver is by Clairitec, made specifically for Medtronic. If you Open the SLDDRW drawing there is a picture of the driver, you can see the ALTERA MAX and EPSON chips.
Currently working on adjusting code for an alternative, 320 x 240,
What is your, alternative for the Display?
Hi there! I’m working with a team that is trying to reproduce the medtronic device. Have you made any progress towards an alternative?
Same for my team no alternative for the blower, display and battery
Sorry, putting this again here if anyone is still interested:
I am working with a mexican team to develop this ventilator, we have been working since march and made a functional prototype using some of the original parts (Display and Turbine). We are facing the same challenges as you on escalating this.
We have succesfully reproduced the battery pack, the PCBs and found subtitutes for valves and turbine, however the display proves to be a challenge.
If anyone is willing to collaborate on this, we would love to share what we have.
We are a mexican team that has been working since March 25th to build this ventilator, by purchasing the (really expensive) Display and original turbine we were able to do our first prototype. LIke you said, the display is a real challenge. We have disassembled the turbine case (it has an air circuit including foam, pretty interesting) and made the CAD file for it, likewise we have found a substitute for the turbine and sensors.
We successfully copied the battery design and PCB,
We are working on an RA8835 alternative along with altering the code for the ST on the CPU but had no luck yet, were you able to code an alternative program for the ALTERA and the ST on the Display Driver? They seem to be protected.
We are fully available to cooperate with any team that wants to build this ventilator, if you have an option for the display, we can completely share the stuff we have.
Hope someone wants to collaborate, reach out.
Hi Vicente
From the information gathered in data in the Medtronic files, we decided to obtain a SP12Q01L6ALZZ display, our plan is to use an Epson driver interface or any suitable controller similar to the Epson. This will make the code changes easier.
We are currently doing calculations/code changes for all the sensors and valve alternatives. we are replacing all Honeywell items.
We just abandoned designing a turbine, and found a all in one replacement turbine module.
Can you provide us with a detailed picture of the turbine interior, in the particular the impeller, and if possible the brush-less DC motor model (we could not make the noise/airflow work).
Thank you
Will look into that.
I Just finished looking through the Maxon website, trying to get a grasp on why the “turbine requirement” document specified a Brushless DC at 72000 rpm for 1 Second.
I am convinced they would use a Maxon motor in this blower and so I wanted to see if they were specifying a no load speed parameter, because the reason they gave for that spec was “impellar adhesion”
Here is an article subject — bench testing ventilators — Turbine vs compressed gas
A bench study of intensive-care-unit ventilators: new …
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2873304
Test lung. Each ventilator was connected to a validated two-chamber Michigan test lung that simulated spontaneous ventilation (Training Test Lung: Michigan Instruments, Grand Rapids, MI). The test lung is composed of two chambers linked by a rigid metal piece.
As far as brushless DC motor and 72000 rpm are concerned — the brushless Dc motor will not produce any electric arcing on a commutator since it doesn’t have one. This is important since there may be a problem when using the device with O2 enrichment. Weird things start to happen when you add O2 – Apollo 1 capsule — Gus Grissom, Roger Chaffee, and Ed White? Anyway — the 72000 rpm for 1 second — since it is an impeller/turbine producing a volume of air (not good at generating pressure) this basically instantaneous burst of volume that can work against the resistance of the patient’s airway and lungs to deliver a quantity of air/O2 to the lungs. At least that’s what I believe is happening. Breathing is a cyclic operation delivering a quantity of air over a period of time — called Inspiration Rate. So the machine will be turning the motor on to simulate inhaling and off to allow for exhaling. I really never understood using turbines in anything else that therapy devices such as CPAP machines used for Sleep Apnea patients, as opposed to volume ventilators using pistons or bellows to drive a quantifiable volume of air at a specific pressure to the lungs. The key words here is “quantifiable volume” which is important because if you cannot verify the volume is reaching the patient’s lungs you are not really helping them. Turbines and propellers are good at flowing air when not acting against an opposing resistance. When the resistance starts to exceed the turbines ability to push air, the turbine/ impeller will start to “cavitate” which means the flow of air stalls or ceases to move. I hope this helps in some way but the thing I don’t get is that I have yet to see some wordage from some medical governing body that informs on the expectations for an ad-hoc ventilation device. I really wish someone to speak to this.
Very interesting article.
I was worried when I saw “compressed air ” in the topic assumed combinations of bellow types and automated valves.
I strayed away from the BVM design frenzy, because I assumed that electronically controlling an ambu bag for expiration (PEEP) would not be easy, and mitigating risk factors for PEEP would be difficult in such a design and therefore not advisable for unassisted ventilation, I’m looking forward to seeing how those projects handle approval requirements.
https://www.gov.uk/government/publications/coronavirus-covid-19-ventilator-supply-specification/rapidly-manufactured-ventilator-system-specification
So glad to see the article used 9 servo-valve and 4 turbine versions.
If I am not convinced of a successful outcome in adjusting the PB560, I think I will look into modifying FreeScales (NXP) ventilator project, to use a compressed gas setup also using FreeScale sensors.
by using ambubag and flow sensor and pressure sensor we can make volume control with pressure limit mode. we can use spi sensor than analog ones
I am working with a mexican team to develop this ventilator, we have been working since march and made a functional prototype using some of the original parts (Display and Turbine). We are facing the same challenges as you on escalating this.
We have succesfully reproduced the battery pack, the PCBs and found subtitutes for valves and turbine, however the display proves to be a challenge.
If anyone is willing to collaborate on this, we would love to share what we have.
With Regards to the PB560 We have compiled the code successfully, meaning it works, however you cannot build the Medtronic PB560, for the following reasons:
1.) You cannot buy the ST10F276 E, however you may be able to get the ST10F276 Z5, the code has been compiled on the “E” also bear in mind ST has stopped “the entire” ST10 range.
2.) The Display is an LCD HMI Module, this was our main concern, because the Clairitec HMI Display module from the pictures seems to have a CPLD, as well as an EPSON LCD controller. From the code you can see that the LCD interface protocols are within the module this means you cannot just update alternative LCD driver code, because ” there is none”.
You will have to re-code the entire LCD portion of the code. In a way this is good, as you can ignore buttons and existing setups and use a touch screen. Or use a different HMI LCD Module the later makes re-coding easier.
Our option was to attempt keeping all LCD coding and re-design a new HMI LCD module writing existing LCD protocols to match, still ongoing.
For us to buy the HMI display module from Clairitec for development debugging, would cost us +/- 60% of a New PB560 ventilator.
3.) There is enough internet chat regarding issues with the Turbine, This may not be a problem, just buy the right BLDC motor and design an Impeller, You can also buy a complete turbine module which interfaces well with the PB560, or a turtle blower similar to airFans turbine (which is used in the PB560) and install your own BLDC, also we noticed airFan also sells a standalone BLDC turtle blower.
4.) Sensors, alot of the sensors on the PB560 are not available, well to us at least. This also is not a problem as the code is very well written and can be changed for different alternatives.
5.) So finally if you want to mass produce you WILL have to re-design the circuit board and entirely re-code, and port to a new MCU, with a new Display solution.
We Have not proceeded with the PB560 Ventilator related research.
If you bypass all the above and build a MEDTRONIC PB560 ……………………you have a problem……………………….. the final hurdle is The code that was written for the PB560, needs to be uploaded using an OEM ST10 Flasher, which inputs most importantly the allocated serial number, without flashing the S/N the program fails at the PUST.
Hope this helps
Our posting above includes an error
Item 1.) ST HAS NOT stopped “the entire” ST10 range as posted above.
We wrote the above in response to emails received
Item 2.) LCD is not ongoing. at the time we were using the MIKROE-2290 TFT LCD Colour Display which has since been aborted.
Not sure about India but in Bangladesh this open sourced Medtronic ventilator, announced in March 2020 has just started being manufactured (April 2021). Too late it would seem for India where hospitals are now overrun and ICU provision is woefully inadequate for the demand. Although a simplified version of a regular ICU ventilator it is still highly complex and seems to require pressurised gas or an oil free oxygen-safe compressor, also challenging to supply in very large quantities. I humbly suggest if a minimum viable spec design had been adopted from the start, vast numbers could have been made by now. How many have actually been made for India? It would be good to know because such decisions have consequences. If you ask medics what they want, they will always give you a high spec. It may be better to have asked what would be a wartime-scenario absolute minimum requirement for 80% of ICU patients, ignoring the remaining 20%. It might have saved more lives in my opinion. https://www.thedailystar.net/business/economy/news/walton-medtronic-ventilators-set-hit-market-finally-2076313