Abstract and Introduction
Abstract
The small but absolute increase in late and very late stent thrombosis associated with drug-eluting stent technology may in part be related to the permanent, durable polymers currently employed with these stent platforms. Research has recently focused on two strategies to combat this problem; bioabsorbable polymers and polymer-free stents. This article critically reviews the key clinical trials and recent conference updates surrounding these two approaches and future directions for technology development.
Introduction
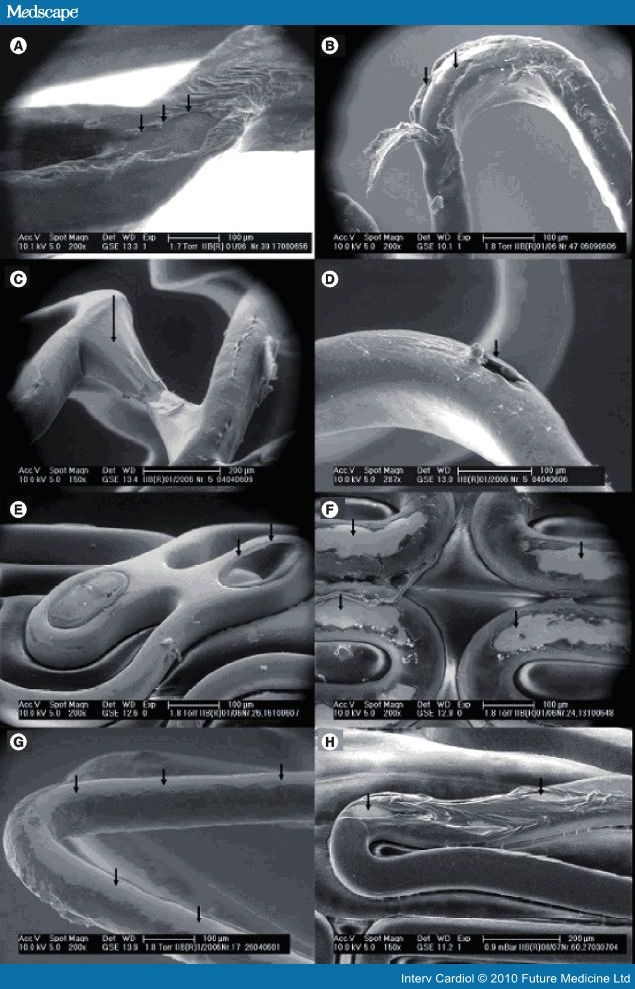
Since their introduction, drug-eluting stents (DES) have revolutionized percutaneous treatment of coronary artery disease with rates of in-stent restenosis of between 2 and 10%[1] and significant reductions in target lesion revascularization.[2] However, there has been a small but important absolute increase in rates of late and very late stent thrombosis (VLST) when compared with bare-metal stents (BMS).[3–5] This increased risk is, in part, due to delayed intimal healing secondary to the antiproliferative drugs employed in current generation stent technology as observed in preclinical,[6] human autopsy, angioscopic and more recently optical coherence tomography (OCT) studies.[7–9] However, the phenomenon is likely to be multifactorial in origin. Clinical and procedural risk factors include overlapping stent zones, stent malapposition, premature discontinuation of antiplatelet therapy, cytochrome P450 2C19*2 loss-of-function allelic variation, diabetes and acute coronary syndrome.[10–13] Increasing concern also surrounds the polymers currently used to bind and control release of drug from stents. Nonerodable permanent (or 'durable') polymers, for example polyethylene-co-vinyl acetate and poly n-butyl methacrylate as used in Cypher® and poly(styrene-b-isobutylene-b-styrene) as used in Taxus™, have been shown to illicit vascular hypersensitivity responses in animal models.[14] Expanded characteristics of contemporary permanent polymer DES are listed in Table 1. In addition, damage to the surface integrity of drug-polymer coating has been described during delivery of durable polymer DES, which may represent a nidus for late thrombus formation (Figure 1).[15]
Figure 1.
Damage to drug-eluting stent surface integrity after failed deployment. (A) Cypher select®, (B) Cypher select, (C) TAXUS Liberté®, (D) TAXUS Liberté, (E) CoStar™ drug-eluting stent (note absence of drug in microwell), (F) Endeavor RX®, (G) Endeavor RX, (H) Xience V™. Reproduced with permission from [15].
These observations have led to several distinct approaches to combat the problems associated with durable polymers. This article examines the clinical trial evidence and recent conference updates accumulated for (i) bioabsorbable polymers that control the release of drug over the short term, but are designed such that after a variable time they have completely degraded leaving only a BMS, and (ii) polymer-free technology that utilizes unique stent design to control drug release.
Interv Cardiol. 2010;2(3):341-350. © 2010 Future Medicine Ltd.
Cite this: Drug-eluting Stents: The Next Generation - Medscape - Jun 01, 2010.