Every time we discuss quantum computers, the headline tends to be that someone, somewhere is going to use the quantum to break your encryption and steal your student loan. If only that were true. But it is probably more realistic to think about quantum computers being used to solve quantum problems. And this has been demonstrated with recent chemistry calculations using a tiny quantum computer.
If solving quantum problems with quantum computers sounds a bit circular, well, it is, but it is also practical. Think of it like this: every protein in your body has the structure it has because of quantum mechanics. And a physicist who is clever, but not intelligent, can write down an exact equation that describes that protein. But not even the most intelligent can solve that equation.
Understanding molecules is hard
A lazier physicist would write a computer script to solve the equation. But that won't work either, because the time it takes to solve an exact description of the molecule will take longer than it takes to go from Big Bang to Heat Death. So we live with approximations. Approximations that are mostly pretty good but sometimes fail spectacularly. And, for some molecules, those approximations don't speed up calculations very much at all.
Quantum computers are supposed to fix this problem. The idea is that if each qubit represents an available state for an electron, then you can build a model of a molecule and let the qubits bounce off of each other until they find the lowest energy state. That would then correspond to the molecule in its most relaxed and happy state.
But that is quite a challenge. Each qubit is a swing, and the swings are all set swinging. If everything is perfect, then the swings all stay in time with each other, and the calculation will work out. However, the noise of the environment causes the swings to stop and start randomly, which changes their timing. This is called coherence, and quantum computers without coherence don't tend to do well. Calculations without coherence often result in nonsense.
So, until the day that we have perfect qubits, even quantum computers have to use approximations.
Let’s play a variation on that game
The optimization process is based on something called the variational principle. It is a very simple principle and very powerful. My molecule has a lowest energy state that corresponds to a particular configuration of electrons and spacings between atoms (or, more accurately, a unique wave function). Now, if I am smart (or, more likely, lucky), I can guess a configuration that is pretty close. The energy for the configuration that I guess will always be larger than (or, at best, equal to) that of the ground state.
The researchers guess a starting configuration. The quantum computer relaxes from the starting configuration to a nearby configuration with the lowest energy. The state and energy are read out and used to contrive a new guess for the ground state configuration.
Repeat that a few times, and, if you are lucky, you'll end up with something close enough to the real ground state. In this case, "close enough" means that you can use the results to accurately predict the properties of the molecule.
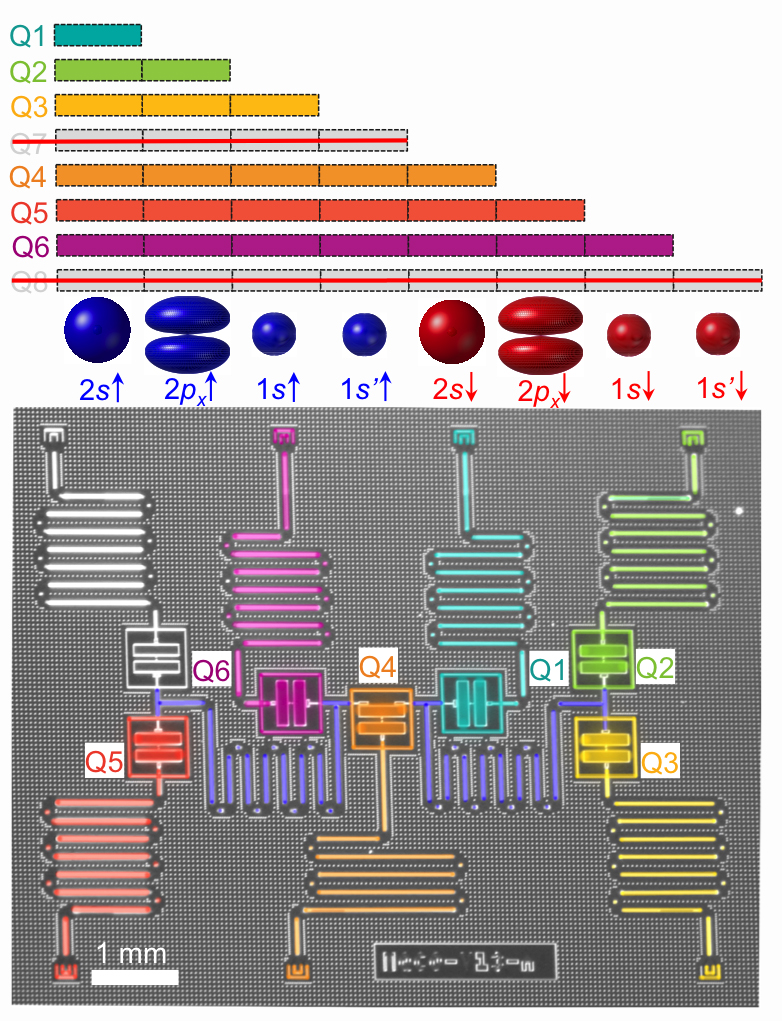
Using a quantum computer with six qubits, researchers were able to set up solvers to obtain the lowest energy state for molecular hydrogen, lithium hydride, and beryllium hydride. This is actually hiding a lot of very cool physics and mathematics. The researchers have come up with a beautifully simple way to represent some rather complicated molecules. The simplicity reduces the computational resources, and it actually is the only reason that the researchers were able to solve for a three-atom molecule on such a small quantum computer.
Beryllium hydride (BeH2) has three atoms and a more complicated electronic structure. That is because beryllium has a full inner shell of electrons, and then the next shell has two orbitals that have very different natures from each other (technically, to model beryllium you need to include an 's' and a 'p' orbital in the calculation). This additional complexity is reflected in the calculations as well. The solution for molecular hydrogen—the simplest structure—is almost perfectly in line with a numerical solution for the full molecule. However, for lithium hydride and beryllium hydride, the solutions are less accurate.
The researchers believe that the falling accuracy is not due to the simplifications but hapens because the qubits do not stay sufficiently coherent during the calculation.
This is not just a guess on the researchers' part, though. Their quantum computer is small enough that it can be modeled using a normal computer. The numerical model was able to reproduce the results of the quantum computer, showing that the researchers understand how their quantum computer is functioning.
And that leads to a small disappointment. Given that, the researchers could have also tweaked the parameters slightly: increased the length of time over which the qubits remain coherent, among other parameters. That would have allowed them to say how much they need to improve their device to obtain an accurate ground state for beryllium hydride, for instance.
Still, as the number of qubits increases, I expect that larger molecules will be modeled, and the accuracy will only get better.
Nature, 2017, DOI: 10.1038/nature23879 (About DOIs).
reader comments
31