Mesenchymal Stem Cells as Vehicles for Gene Delivery
MSCs seem to be receptive to transduction with various viral vectors, including adenovirus, adeno-associated virus, retrovirus, herpes simplex virus, lentivirus and spumavirus (also termed foamyvirus) ( Table 1 ), so it is conceivable that some of the aforementioned limitations of current OA therapies might be overcome by adaptation of MSC-based gene-transfer technologies.[50] This approach will involve isolation of MSCs, ex vivo genetic modification of the MSCs, and transplantation of the modified cells into the diseased joint.
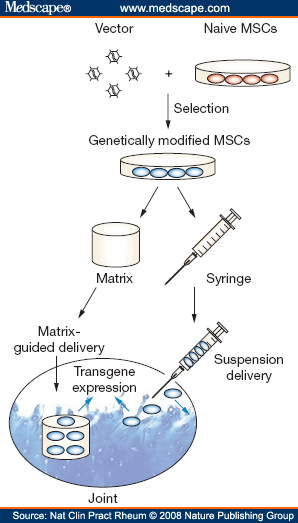
Generally, ex vivo gene-delivery approaches are more invasive, expensive and time-consuming than in vivo approaches (in which therapeutic vectors are applied directly into the body), but they do permit control of the transduced cells and safety testing before reimplantation.[51] In particular, use of MSCs should allow the development of techniques for delivering genes that encode proteins that might reverse some of the major pathologies of OA ( Table 2 ).[13,51] Analogous to the delivery approaches described above for native MSCs (Figures 1a and 1b), genetically modified MSCs can be delivered to joints either as a cell suspension to counteract the inflammatory and matrix degradation processes, or via matrix-based strategies to induce formation of neocartilage tissue (Figure 3).
Figure 3.
MSCs Can Be Used as Vehicles for ex vivo Gene Delivery. Cell-based approaches to osteoarthritis therapy might be augmented by use of genetically modified MSCs, which would involve gene transduction of culture-expanded MSCs. Successfully transduced cells would be isolated and applied to the joint space either as a cell suspension, or seeded within a biological matrix that can be implanted in a cartilage defect. Depending on which delivery approach is chosen, ubiquitous or local transgene expression is induced by the genetically modified MSCs, and the gene products could beneficially influence osteoarthritis pathology. Abbreviation: MSC, mesenchymal stem cell.
Delivery By Cell Suspension
Following delivery of cell suspensions, the aim is for transduced MSCs to release therapeutic proteins that interact with all available tissues, including cartilage. Considerable progress has been made towards defining the parameters that prolong intra-articular transgene expression, an approach that was originally developed for the treatment of rheumatoid arthritis (RA).[52] Current research suggests that immunologically compatible vector systems allow sustained intra-articular transgene expression.[53] In a phase I clinical study, IL-1 receptor antagonist complementary DNA was successfully retrovirally delivered by an ex vivo strategy to the metacarpophalangeal joints of individuals with RA.[54] This study shows that genes can indeed be delivered safely to human joints, and highlights the clinical utility of ex vivo gene transfer as a treatment for arthritis.[55] Data are beginning to emerge on the potential of such an approach for treating OA; encouraging results have been reported for IL-1 receptor antagonist adenovirally delivered to the joints of horses with experimental OA.[56] Furthermore, insulin-like growth factor 'administered' by intra-articular delivery partially reversed matrix degradation in OA.[51,57,58] Other cell types were initially investigated, but MSCs have the potential to be at least as beneficial when used in ex vivo approaches.[13,16,59]
A growing body of literature indicates that many of the pleiotropic gene products considered necessary for cartilage repair and regeneration are compatible with intra-articular delivery in suspension. However, delivery of transforming growth factor β1 or bone morphogenetic protein 2 to the synovium resulted in severe swelling, fibrosis, and osteophyte formation within joints.[60,61] Candidate complementary DNAs for synovial gene transfer should, therefore, be carefully chosen, safety-tested and validated ( Table 2 ).
Delivery Within a Matrix
The above-mentioned anti-inflammatory treatments for RA and OA are, in principle, useful for preventing disease progression, but might not be able to restore damaged cartilage. An alternative strategy uses genetically modified MSCs in matrix-guided approaches to cartilage regeneration.[59,62] MSCs are first stimulated to undergo chondrogenic differentiation, stabilized as chondrocytes, then introduced on a matrix to the defect site, with the aim of establishing a cartilage phenotype without progression to hypertrophy or dedifferentiation.[13] A number of in vitro systems that use various transgenes ( Table 2 ) demonstrate that MSCs can undergo chondrogenesis efficiently in defined, three-dimensional, serum-free, culture conditions.[44] Data indicating that delivery and expression of certain genes might bias the repair response towards the synthesis of normal articular cartilage in vivo are beginning to emerge.[59] As already mentioned, however, this approach has been used mainly to treat focal cartilage defects. Future studies will show whether such technology will be suitable for repairing large areas of eroded cartilage, as occurs in advanced OA.[63]
Nat Clin Pract Rheumatol. 2008;4(7):371-380. © 2008
Nature Publishing Group
Cite this: Technology Insight: Adult Mesenchymal Stem Cells for Osteoarthritis Therapy - Medscape - Jul 01, 2008.














Comments