Miniaturization of mechanical motors, actuators, and electronic components saves lives in a small but significant way.
April 15, 2021

Moore’s Law has been the main driver in the miniaturization of electronics. Material science and advanced semiconductor manufacturing techniques have played a significant role in the shrinking of motors and actuators. Taken together, all of these advances have led to the development of mini-actuators, component miniaturization, and medical coating materials for many types of medical devices.
Two critical areas of medical miniaturization have been in the ingestible and implantable areas. Let’s consider ingestible technology first.
Ingestible Devices
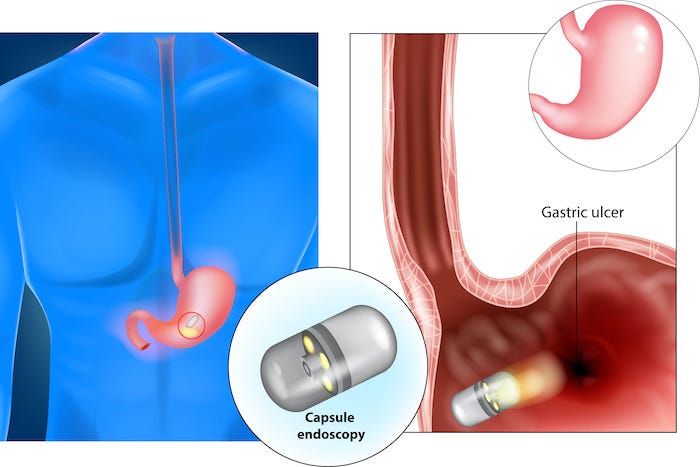
For many decades, researchers have shared their development advancements of tiny ingestible pills filled with cameras, sensors, and electronics that could travel in the human gastrointestinal (GI) tract.
Sometimes known as ingestible wireless capsule endoscopy (WCE), these smart pills serve as a diagnostic technology for inspecting the entire GI tract for various diseases, including internal bleeding, tumors, cancer, and other conditions. According to the report by “Markets & Markets,” the smart pills business is forecast to reach $ 3.83 billion in 2020, growing to reach $ 8.98 billion by 2024.
Highly precise motors and actuators encounter unique challenges when operating inside a biological system. In the medical space, actuators are used to move micro-optics and adjust medical imaging lenses, lasers, and other tiny mechanical components. Other applications could include medicine dispersants, robotic surgical tools, and more.
Developers of miniaturized motor and actuators are eager to learn if their devices might work inside a tiny ingestible pill to collect samples as the capsule travels through the GI tract or to help dispense drugs at the right moment and place in the body.
Other potential applications include using micro-actuators to spin a mirror to provide a 360-degree view for the cameras as the pill passes through the GI tract, providing the information in a camera form, or acting as an optical coherence tomography (OCT).
But have any of the research-level micro-actuator pill applications made it into the commercial space, say, for pill endoscopy? If so, do any use actuators?
After some discussion, it turned out that existing micro-actuators and motors were not just scaled-down versions of larger motors but rather based on piezoelectric principles that use ultrasonic vibrations for motion. These tiny devices – some as small as a grain of rice – are based on the control of vibrations above audible sound, i.e., 20K Hertz. When a vibrating body touches a moveable object, then the motion can be generated. These devices are typically used for diagnostic and applications outside the body – not inside it as with a pill.
One actuator expert explained that these are mesoscale, millimeter microsystem devices for applications in micro-sensing, actuator-induced motion, and fluidics. They are not based on microelectromechanical systems (MEMS) technology, which was typically designed for very low-power apps like switching a mirror or aiming a laser.

Pills with Motors?
Are any ingestible pills (with or without actuators) actively in use by today’s doctors? One example is Medtronic’s Pillcam, a vendor in the commercial space that had been supplying the technology for several years.
A doctor does need to prescribe an ingestible pill. After a patent swallows the pill, a camera records the information as it moves through the GI tract to be accessed later or provided wirelessly in real-time during the activity.
Newer versions of the PillCam might be steerable. In fact, there are already categories of operation to include steerable pills. These categories include wearable, plantable (in the stomach), and even passible (through the body) devices. In the latter category, only the patient’s digestive system is exposed, which means it was really a type of endoscopy.
One question that arises is that if a micro-motor could be implanted as a steering mechanism, would it be able to withstand the fluids in the human body? Some experts say “no,’ that current micro-motor devices have to be kept away from body fluids as there would be moving mechanical parts and electronics components that might leak into the body.
Similarly, any camera would have to be free of any body fluids, which is why cameras are encapsulated in an ingestible pill behind a window. Several companies are in the market of developing coatings to encapsulate cameras and electronics. These coatings are typically 5 to 20 microns in thickness and are optically clear.
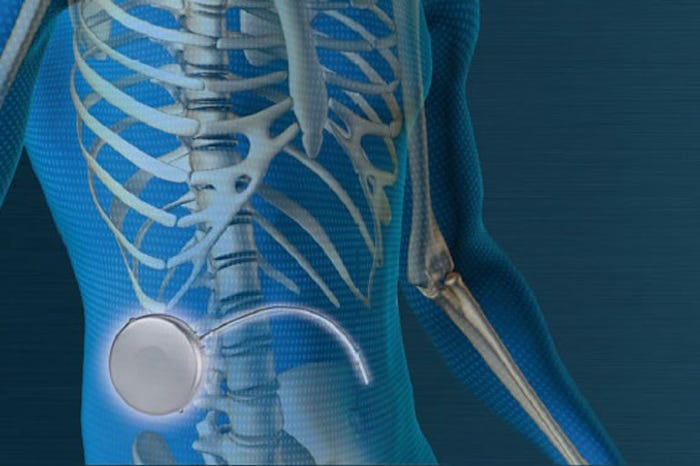
Implantable Devices
Implantable medication delivery systems allow targeted drug delivery at specific rates that may minimize potential side-effects of therapy. Where such delivery systems are vital for the survival of the patients, the systems have to be absolutely reliable. A key element of the implanted delivery system is high-precision microdrives.
Patients who suffer from chronic pain, metabolic disorders, diseases of the central nervous system, and tumors require accurately dosed medication. Implantable medication delivery systems provide a precise and reliable method to achieve that delivery. For many patients, it is only the medication delivery system that allows them to have a life outside of the clinic.
As the name suggests, implantable devices are inserted into the human body, typically just under the skin. The location is well suited to deliver precise medication dosage at defined times during the day.
In a wireless implementation, the implantable unit has an RF data interface to the embedded device by which the dosage can be adapted at any time. At regular intervals, the internal medication reservoir can be refilled by a specialist. The life span of the active implants measures many years and is limited only by the life of the battery. The core of the active implant is a piston pump, such as those manufactured by Maxon Medical.
One design challenge with these implantable medication delivery system microdrives is that they are not compatible with ordinary motors. In a customary motor, rotary motion is generated and yields torque and speed of rotation. The reciprocating piston pump generates a linear movement that results in liquid pumping, notes a Maxon application article. The individual parts of the pump have very narrow tolerances and allow the pump volume to be adjusted with a precision of less than a microliter per piston stroke.
Further, as active implants are in direct contact with human tissue, biocompatible materials are an absolute requirement. Therefore, in most cases, titanium is used for implants. Machining titanium, especially safe and reliable laser welding, requires a very high level of expertise. Special testing and quality methods, such as inspecting the penetration depth, microhardness, and density of the weld seams is required. The entire assembly of the implantable microdrive typically takes place under cleanroom conditions.
John Blyler is a Design News senior editor, covering the electronics and advanced manufacturing spaces. With a BS in Engineering Physics and an MS in Electrical Engineering, he has years of hardware-software-network systems experience as an editor and engineer within the advanced manufacturing, IoT and semiconductor industries. John has co-authored books related to system engineering and electronics for IEEE, Wiley, and Elsevier.
About the Author(s)
You May Also Like





