Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Coatings
Fluorinated coating is utterly repellent
Transparent, self-healing film fends off more than 100 liquids, including concentrated acids and low-surface-tension solvents
by Mark Peplow, special to C&EN
October 16, 2018
| A version of this story appeared in
Volume 96, Issue 42

Some liquid-repelling coatings stay dry in a deluge of water, while others rebuff oils and organic solvents. Some can withstand harsh treatment, shrugging off harsh acids, high temperatures, or even healing themselves after being damaged.
Now researchers have created a veritable Swiss Army knife of a repellent coating that combines all of these properties and more, thanks to its unique chemistry and texture (Nat. Mater. 2018, DOI: 10.1038/s41563-018-0178-2). “We’re controlling a range of different structures, from the chemical bond to the nanoscale to the microscopic level,” says Frank Caruso of the University of Melbourne, part of the team behind the super-omniphobic material.
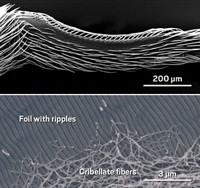
The coating is a mixture of 1H,1H,2H,2H-perfluorohexyltrichlorosilane (PFTS) and n-butyl cyanoacrylate (n-BCA), combined in a dichloropentafluoropropane solution. When sprayed onto a surface, water vapor in the atmosphere triggers a series of polymerization reactions between PFTS and n-BCA to create a tough, transparent film. The coating contains polymer nanoparticles that aggregate to form a highly textured surface, which may trap tiny pockets of air to help ward off liquids.
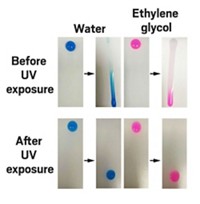
In tests, the coating repelled more than 100 different liquids, including water, n-pentane, perfluorohexane, and concentrated hydrofluoric acid. Droplets of liquid typically formed almost spherical globules on the coating, with contact angles of at least 150 degrees—generally regarded as the mark of a super-repellent surface. Slightly tilting a surface—typically less than 5 degrees—was enough to make the droplets roll off cleanly. A jet of n-pentane, which has a very low surface tension and will wet most surfaces, simply bounced off coated surfaces.

The researchers say that n-BCA acts as a powerful adhesive to anchor the coating to a wide range of substances, including wood, metal, glass and polyester fabric. Caruso suggests that this strong adhesion may also reduce the risk of the coating escaping into the environment, a problem that has bedeviled other polyfluorinated compounds.

The coating continued to repel liquids at 100 °C and retained its properties after scratching, abrading, and washing. Only when the researchers scoured it with oxygen plasma did it lose its repellency. Even then, the material healed itself after 24 hours at room temperature, or in just 10 minutes if heated to 120 °C, regaining its full complement of super powers as its polymer chains reorganized themselves.


Many of these properties have been combined in previous materials, says Doris Vollmer at the Max Planck Institute for Polymer Research, who studies super-repellent coatings. But the coating’s ability to repel n-pentane is unusual, as is the complete recovery of n-pentane repellency after self-healing. “That is better than I’ve seen with other coatings,” she says.
The simple, spray-on method could also make this coating attractive for commercial applications, says Jas Pal S. Badyal, who develops functional surfaces at Durham University. “The one-step approach is good,” he says. However, using dichloropentafluoropropane as a solvent for the coating’s precursors could be a stumbling block, he adds, due to the environmental impact of such halogenated compounds.
Advertisement
Caruso suggests that the material could eventually be developed into a coating for chemical hazard shielding, and the team is already working with industry partners. “But there’s still a long way to go in terms of applications,” he adds, noting that they need to reduce the cost of the coating and assess its long-term durability.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter