Hair follicle stem cells and their daughter cells participate in a regenerative cycle that can slow to a halt if too few daughter cells fail to recapture their “stemness,” that is, if they fail to reverse their fate and become stem cells once again. Although daughter cells are needed to generate the hair follicle’s outer root sheath, a pool of stem cells must be maintained. Otherwise, cells of the hair follicle suffer exhaustion and perish. And when that happens, hair loss follows.
The root issue, reported researchers from Cologne and Helsinki, is metabolic. When cells change their stem cell state, they change their metabolic state. And it happens that hair follicle stem cells are metabolically adept at tolerating low-oxygen conditions beneath the skin’s surface. The daughter cells, or the progenitor cells, are less so. They rely not on glycolysis, as do stem cells, but on oxidative phosphorylation and glutamine metabolism. According to the researchers, preventing hair loss requires that cells remain flexible metabolically. They must be able to switch back to glycolysis.
This finding was described in Cell Metabolism, in an article titled, “Glutamine Metabolism Controls Stem Cell Fate Reversibility and Long-Term Maintenance in the Hair Follicle.” The paper also explained how the metabolic state of the cells could be manipulated.
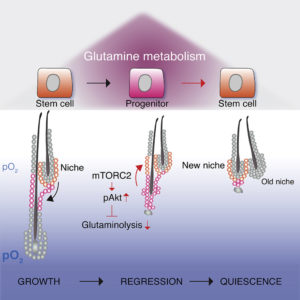
“Hair follicle stem cell (HFSC) fate reversibility and glutamine metabolism are regulated by the mammalian target of rapamycin complex 2 (mTORC2)-Akt signaling axis within the niche,” the paper’s authors wrote. “Deletion of mTORC2 results in a failure to re-establish the HFSC niche, defective hair follicle regeneration, and compromised long-term maintenance of HFSCs.”
Every day, tissues such as the skin and its hair follicles are exposed to environmental damage like ultraviolet radiation. Damaged material is continuously removed and renewed. On average, 500 million cells and 100 hairs are shed every day, amounting to 1.5 g of material. The dead material is replaced by stem cells, which are specialized, highly proliferative, and long-lived. Tissue function relies on the activity and health of these stem cells; compromised function or reduced number leads to aging.
“Although the critical role of stem cells in aging is established, little is known about the mechanisms that regulate the long-term maintenance of these important cells,” said Sara Wickström, MD, PhD, one of the study’s senior authors and a researcher affiliated with the University of Helsinki and the Max Planck Institute for the Biology of Aging. “The hair follicle, with its well-understood functions and clearly identifiable stem cells, was a perfect model system to study this important question.”
Wickström and the study’s other senior author, the University of Cologne’s Sabine Eming, MD, PhD, led a scientific team that sought to understand what made stem cells functionally distinct from their differentiated daughter cells. Ultimately, the team investigated the transcriptional and metabolic profiles of the two cell populations.
“Intriguingly, [we found] that stem cells and daughter cells have distinct metabolic characteristics,” said Christine Kim, PhD, of the Max Planck Institute for Biology of Ageing and one of the study’s two lead authors of the study. “Our analyses further predicted that Rictor, an important but relatively poorly understood molecular component of the metabolic master regulator mTOR pathway, would be involved.” The mTOR signal transduction regulates processes such as growth, energy, and oxygen consumption of cells.
In more detailed analyses, the team showed that stem cell depletion was due to the loss of metabolic flexibility. At the end of each regenerative cycle, during which a new hair is made, the stem cells will return to their specific location and resume a quiescent state.
“The key finding of this study is that this so-called fate reversibility requires a shift from glutamine metabolism and cellular respiration to glycolysis,” stressed Xiaolei Ding, PhD, of the department of dermatology, University of Cologne and the study’s other lead author. “The stem cells reside in an environment with low oxygen availability and thus use glucose rather than glutamine as a carbon source for energy and protein synthesis.
Ding and Eming had recently generated a genetic mouse model to study Rictor function and observed that mice lacking Rictor had significantly delayed hair follicle regeneration and cycling, which indicated impaired stem cell regulation.
“Interestingly,” noted Ding, “with aging, these mice showed hair loss and reduction in stem cell numbers.”
“A major future goal will be to understand how these preclinical findings might translate into stem cell biology in humans and potentially could be pharmaceutically harnessed to protect from hair follicle aging,” said Eming. “We are particularly excited about the observation that the application of a glutaminase inhibitor was able to restore stem cell function in the Rictor-deficient mice, proving the principle that modifying metabolic pathways could be a powerful way to boost the regenerative capacity of our tissues.”



