Extracellular materials may become integral to device production. OEMs should familiarize themselves with those on the market and in the pipeline.
TISSUE ENGINEERING
|
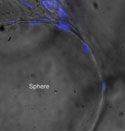
Figure 1. (click to enlarge) Fibroblasts (blue DAPI stain) cultured on bead-like sphere structures produce embryonic ECM. |
Tissue engineering and regenerative medicine use various scaffolds, living cells, and cell-based products. Many cell-based therapies on the market are testaments to the clinical benefits of such therapeutics. However, these products present challenges. Some of the challenges involve cell retention or engraftment, packaging and shelf life, the regulatory approval process, and reimbursement.
In contrast, products derived from human cells, such as a naturally secreted extracellular matrix (ECM), offer an alternative to living cells in a final product. This article describes a human extracellular matrix (hECM) material that is manufactured to mimic characteristics of the embryonic environment.
The hECM is grown through a platform tissue-engineering technology using neonatal fibroblasts, which create a nonsoluble matrix material, similar to the embryonic structural tissue that provides the framework for development of blood, skin, muscle, and bone (see Figure 1).
|
Figure 2. A scalable bioreactor system creates fibroblast. |
Creation of this material begins by using a scalable bioreactor (see Figure 2) to seed newborn fibroblasts onto microcarriers. They are grown in suspension while being conditioned with liquid media. Within a few days, under hypoxic culture conditions that simulate the embryonic environment, the cells produce a dense embryonic-like extracellular matrix and secrete wnt proteins (molecules that regulate cell-to-cell interactions in embryogenesis) and various growth factors into the media.
Human ECM does not carry the same risk of an allergic reaction or the transmission of animal viruses as do porcine and bovine tissues. The material can be used as a coating for implants to improve tissue ingrowth, vehicles for cell delivery, injectables for soft-tissue augmentation, and tissue regeneration patches.
Tissue-Engineering Challenges
Tissue engineering creates substitutes that can repair or replace damaged or diseased tissues and organs. Unlike traditional devices, tissue constructs are engineered to remain biointeractive after implantation, thereby offering the structure and physiologic functions of the replaced tissue.
To date, three fibroblast-based living products have been FDA approved for use in treating hard-to-heal wounds. One example, Dermagraft, a living human dermal implant, was invented to provide living cells, human matrix components, and growth factors to wounds.1,2 Dermagraft products are manufactured using neonatal dermal fibroblasts from routine circumcisions. Such cells offer reproducibility (cells are always the same age, sex, and from the same anatomical location) and expansion potential (one sample yields cells sufficient for 250,000 sq ft of Dermagraft).
In terms of regulatory and safety standards, Dermagraft goes through rigorous processes. Cells are extensively tested for known viruses and pathogens at several stages. Manufacture of the dermal implant occurs in a completely closed bioreactor system. The system maintains sterility while mimicking a physiological environment in which cells, seeded onto biocompatible three-dimensional scaffolds, divide and secrete all human growth factors and matrix proteins to form a functional tissue. In-process testing is performed to measure sterility, cell activity, and tissue formation while closely monitoring oxygen concentrations, pH, temperature, and nutrient uptake. The same bioreactor is used to seed the cells and grow, freeze, and store the tissue.
The development of cell-based tissue engineering taught researchers many important lessons about how to manufacture and ship such products. It also highlighted the unique clinical, regulatory, and reimbursement processes that cell-based products face.
Key takeaways were the benefits of using highly tested cell banks from newborn foreskins as well as the need for closed manufacturing systems, tight in-process controls, relevant release criteria, and product preservation. Due to normal biologic variation, however, manufacture and release of living products are expensive and rejection rates are high. In addition, proper product handling prior to implantation is critical for viability.
Living products have shown an ability to adapt to the wound environment. For example, fibroblast-based products placed in an ischemic environment produce high quantities of various angiogenic factors to induce new blood vessel growth, a finding that led to the development of Anginera for the treatment of conditions associated with ischemic heart muscle.3,4
In addition, the ECM that is deposited by the cells both during the manufacturing process and in vivo helps to naturally fill in the wound bed and induce regeneration.
The ability of cells to respond to their environment, along with the clinical benefits seen with hECM, led to the development of new manufacturing processes using a scalable, closed hypoxic bioreactor system.
The system supports the production of a human matrix that closely resembles an embryonic matrix. Embryonic or fetal ECM is thought to play an important role in fetal wound healing, which occurs with little or no scar formation.5 Such minimal scar formation led researchers to explore growing neonatal fibroblasts under fetal-like conditions to encourage the production of hECM and conditioned media with fetal-like characteristics. In the new bioreactor system, neonatal fibroblasts are grown and cultured to create hECM and conditioned medium.
The hECM and conditioned medium are by-products of the culture process and are being evaluated for a variety of clinical and research uses. Because these two products do not contain living cells, there is minimal need to have close controls for maintaining a specific level of cell viability at product release, long-term storage, and product handling at time of use. The hECM can replace animal collagens and ECM products.
Coatings for Tissue Ingrowth
Within the medical device industry, numerous manufacturers have used ECM and ECM components (collagen) to treat or modify the surface of a device. Familiarity with surface coatings and surface modifications already exists within the device industry.
Surface-modification efforts are used to alter the way the body reacts to the surface of a device and therefore alter or influence wound healing. In this way, a coating can help promote a favorable healing response and increase biocompatibility.
Typical sources for ECM are animal products, either bovine or porcine; these offer several benefits. The sources are readily available as by-products of the meat-packing industry. They are cost-effective because highly technological processing isn't required and because they are broadly used.
Collagen is the most widely used ECM protein for coating the surface of medical devices. Collagen coatings have been used in vascular grafts. Additionally, the Finale Prohealing coating from Surmodics, which uses a collagen-1 and laminin-1 blend, has been evaluated as a surface treatment for stents to improve biocompatibility.
However, animal-sourced tissue has limitations. In some patient populations, certain allergies, religious beliefs, or ethical considerations can hinder clinical applications. In some countries, medical devices that contain animal-based tissues or products are not allowed under any circumstances. Therefore, the future development of surface coatings may be directed toward a nonanimal source of ECM or specific proteins.
A human acellular source may be able to meet future medical device requirements. For example, hECM has demonstrated rapid host-cell integration, the elimination of immunogenicity, and a reduction in erosion of host tissues. Because the hECM is derived from neonatal cells that have been tested for all known viruses and pathogens, concerns regarding viral and disease transmission from human cadaveric tissues (TSE) and animal tissues (BSE) are limited. A highly controlled closed manufacturing system produces a consistent product composition and performance compared with cadaveric dermis and fascia lata.
|
Figure 3. (click to enlarge) Exposure of human fibroblasts to hECM induced a dose-dependant increase in cell number as measured by the Pico Green assay. (FCS stands for fetal calf serum.) |
The hECM material has been evaluated using a variety of bioassay techniques. Using in vitro evaluation, the material was seeded with human fibroblasts and compared with a mouse ECM material equivalent. The human fibroblasts were found to preferentially migrate into the hECM material and take residence while maintaining normal morphology. Additionally, when mouse and human ECM materials were compared, a dose-dependent increase in cell number was associated with exposure to increasing concentrations of the hECM material as measured by the Pico Green assay (see Figure 3).
|
Figure 4. (click to enlarge) Human ECM implanted onto a chick chorioallantoic membrane (CAM) stimulated a microvascular response. New microvasculature is depicted with arrows. |
The chick chorioallantoic membrane (CAM) assay has also been used to test the response of the hECM material on a very sensitive in vivo membrane. With implantation of the hECM, the membrane remained transparent and did not thicken. Such results indicate a favorable in vivo response to the material. Furthermore, a microvascular response was noted within the hECM material after implantation into the CAM assay (see Figure 4).
Cell Retention and Engraftment Challenges
Many device manufacturers and medical therapy companies have invested in using living cells as therapeutic agents to deliver certain products (e.g., growth factors) or to act as replacement cells for lost tissues. These therapies require that cells maintain activity at the transplant location whether in bone marrow, skin, the liver, or the heart.
In the field, such viability has proved a significant hurdle. For example, the best published results to date for the beneficial effects of bone marrow cell transplantation after acute myocardial infarction come from the BOOST trial.6 In BOOST, significant improvements in left ventricular ejection fraction were seen six months after treatment with bone marrow cells. However, these improvements were no longer significant at 18 months.
A few investigators have reported cell retention as a major obstacle for cell replacement therapies, indicating that only 1.3–2.6% of infused bone marrow cells are retained in the heart.7 Although cell-based therapies continue to advance through clinical trials, many investigators are looking to delivery agents such as hydrogels, alginates, and polymer scaffolds to first provide a hospitable environment for transplant cells so that the cells can be delivered into the host tissue. In this area, hECM material may introduce a platform-enabling technology.
Human ECM can have embryonic-like characteristics and therefore enable culturing of transplant cells, especially adult or embryonic stem cells. Using an hECM material as a delivery vehicle for these therapies may facilitate improvement in engraftment and long-term outcomes.
Injectables
Several devices are currently available as injectables for soft-tissue replacement and augmentation and as bulking agents to treat gastroesophageal reflux disease (GERD) and urinary incontinence. These products are either permanent, such as silicone, or temporary (requiring administration every 4–6 months).
Historically, the most commonly used injectables have been cross-linked bovine collagen derived from cowhide from closed herds. In 2003, Inamed substituted bovine collagen with a tissue-engineered human collagen and created CosmoDerm and CosmoPlast. This substitution was regulated as a raw material change by FDA and created a product that did not require the two-week skin test that is necessary for bovine derived materials.
Another example, Cymetra, is a micronized particulate injectable human extracellular matrix derived from cadaver dermis. Cymetra contains collagens and elastin, to provide structure for cell repopulation, and proteoglycans and proteins that allow the patient's own cells to initiate revascularization and cell repopulation.
Hyaluronic acid, a natural sugar that binds to water to create temporary volume, has gained increasing popularity in treating wrinkles and contour defects. Currently, there are no injectable materials on the market that replace all naturally occurring matrix components including collagens, hyaluronic acid, fibronectin, and other glycosaminoglycans.
Tissue-engineered hECM contains many of the matrix proteins that are found in young skin. For example, the hECM produced under hypoxic culture conditions contains collagens type III and V; glycoproteins such as fibronectin, SPARC, and thrombospondin; and hyaluronic acid. Therefore, hECM material has the potential for use as a treatment for wrinkles and contour defects, and as a bulking agent.
ECM Regeneration Patches
Several devices have been developed based on animal and human extracellular components that have demonstrated clinical effectiveness in promoting tissue regeneration. Integra Dermal Regeneration Template is a bilayer membrane system for skin replacement in partial- and full-thickness burns. The dermal replacement layer is made of a porous matrix of fibers of cross-linked bovine tendon collagen and a glycosaminoglycan (chondroitin-6-sulfate). It is manufactured with a controlled porosity and defined degradation rate. ECM materials in Integra support migration of the patient's cells into the product and the regeneration of a dermis.
Surgisis Biodesign is a tissue repair product manufactured from decellularized porcine intestinal submucosa. Its ECM components signal surrounding tissue to grow across the scaffold, allowing the body to restore itself. Surgisis Biodesign is approved for hernia repair, fistula repair, plastic and ENT reconstructive surgery, staple line reinforcement, Peyronie's repair, continence restoration, dural repair, and pelvic floor repair.
A similar product from ACell is derived from porcine urinary bladders and referred to as UBM (urinary bladder matrix). ACell's ECM is a naturally occurring, completely resorbable, non-cross-linked, acellular biomaterial. ACell has five 510(k) approvals in a broad range of clinical settings including wound and general surgical applications.
An hECM product that has a variety of clinical uses is Alloderm. Alloderm is a decellularized hECM derived from cadaver dermis. Since its composition is the same as the donor skin, Alloderm is regulated as a tissue-bank product. It contains human collagens, elastin, and proteoglycans and is used clinically for cutaneous wounds, hernia repair, periodontal applications, and breast augmentation.
Another product, TransCyte, is a tissue-engineered hECM with premarket approval for partial- or full-thickness burns. The ECM is created by seeding neonatal fibroblasts onto a Biobrane scaffold placed within a closed bioreactor system that supports the uniform deposition of human collagens and glycosaminoglycans during a three-week manufacturing process. At the end of the process cells are lysed and the product is rinsed before being frozen. Trancyte induces rapid epithelialization and has shown statistically significant fast healing, pain reduction, and reduction in care time for middermal facial burns.8
Using such product knowledge, the tissue-engineering community should soon offer new advancements. For example, bioreactors have been developed to expose neonatal fibroblasts to early embryonic conditions during the production of ECM and conditioned media. The resulting hECM contains a protein matrix that includes collagen I, tenacin, hyaluronic acid, and fibronectin, as well as proteins that have been described to be prevalent in embryonic ECM, such as collagens III, V, and SPARC. Embryonic matrix components are known to be associated with scarless wound healing and may prove beneficial in accelerating wound healing and tissue regeneration.
Research Tools and In Vitro Applications
Significant progress continues in the development of new diagnostics and next-generation devices. Many new cell lines are being evaluated for their potential therapeutic use.
An hECM material in this field enables improvement in existing techniques and development efforts using regenerative cells such as adult and embryonic stem cells. This material is created as a nonsoluble matrix, most similar to early embryonic structural tissue. By producing ECM materials similar to those that helped guide the original formation of body parts in the embryo, regenerative cells are supported in vitro and potentially as therapeutics in vivo. A key requirement for culturing human embryonic stem cells is an ECM that can be used to coat culture dishes. Current ECM products used for these applications are animal-based. 9–11
|
Figure 5. (click to enlarge) Embryonic stem cells were grown and expanded on the hECM material. |
The hECM material has been evaluated as a substrate for the growth of embryonic stem cells. Current culture techniques for the growth and expansion of embryonic stem cells require the use of a mouse ECM that is derived from tumors. As such, it presents challenges for translating into a clinical application. In recent studies, embryonic stem cells were grown and expanded on the hECM while maintaining their pluriopotent morphology (see Figure 5). These embryonic cells preferentially targeted the hECM and did not attach to the areas of bare beads.
Conclusion
The development of a manufactured hECM material without the presence of living cells in the final product, effectively addresses challenges with previous tissue-engineered products that contain living cells.
Device manufacturers should explore the developments provided by tissue-engineered products. Familiarity with the challenges and benefits of these materials helps foster blending device and bioengineering disciplines.
Gail Naughton is the Dean of Business Administration at San Diego State University and CEO of Histogen Inc. (San Diego). She can be reached at [email protected]. Robert Kellar also works at Histogen and can be reached at [email protected].
References
1. J Mansbridge et al., “Growth Factors Secreted by Fibroblasts: Role in Healing Diabetic Foot Ulcers,” Diabetes, Obesity, and Metabolism 1, no. 5 (1999): 265.
2. G Naughton, J Mansbridge, and G Gentzkow, “A Metabolically Active Human Dermal Replacement for the Treatment of Diabetic Foot Ulcers,” Artificial Organs 21 (1997): 1203.
3. RS Kellar et al., “Scaffold-Based, Three-Dimensional, Human Fibroblast Culture Provides a Structural Matrix That Supports Angiogenesis in Infarcted Heart Tissue,” Circulation 104, no. 11 (2001): 2063.
4. RS Kellar et al., “A Cardiac Patch Constructed from Human Fibroblasts Attenuates a Reduction in Cardiac Function Following Acute Infarct,” Tissue Engineering 11 (2005): 1678.
5. NS Adzick and HP Lorenz, “Cells, Matrix, Growth Factors, and the Surgeon. The Biology of Scarless Fetal Wound Repair,” Annals of Surgery 220 (1994): 10–18.
6. GP Meyer et al., “Intracoronary Bone Marrow Cell Transfer after Myocardial Infarction: Eighteen Months' Follow-up Data from the Randomized, Controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) Trial,” Circulation 113, no. 10 (2006): 1287–1294.
7. M Hofmann et al., “Monitoring of Bone Marrow Cell Homing into the Infarcted Human Myocardium,” Circulation 111 (2005): 2198–2202.
8. RH Demling and L DeSanti, “Management of Partial Thickness Facial Burns (Comparison of Topical Antibiotics and Bio-engineered Skin Substitutes),” Burn 25 (1999): 256–261.
9. D Rajesh et al., “Differential Requirements for Hematopoietic Commitment between Human and Rhesus Embryonic Stem Cells,” Stem Cells 25, no. 2 (2007): 490–499.
10. JC Mohr, JJ de Pablo, and SP Palecek, “3-D Microwell Culture of Human Embryonic Stem Cells,” Biomaterials 27, no. 36 (2006): 6032–6042.
11. JM Fletcher et al., “Variations in Humanized and Defined Culture Conditions Supporting Derivation of New Human Embryonic Stem Cell Lines,” Cloning Stem Cells 8, no. 4 (2006): 319–334.
Copyright ©2008 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like