Martin Heywood
Spirit Energy Ltd.
Lancaster, UK
Matthew Uhe
Spirit Energy Ltd.
Barrow-in-Furness, Cumbria, UK
Tobias Eckardt
BASF Catalysts Germany GMBH
Nienburg, Germany
Margaret Greene
BASF Corp.
Iselin, NJ
Ray Racher
BASF Corp.
Baton Rouge, La.
Roger Wyatt
BASF SE
Birmingham, UK
In 1994, British Gas PLC commissioned a natural gas processing plant at the North Morecambe onshore terminal at Barrow-in-Furness, Cumbria, UK, based on a novel combination of existing technologies using adsorbents from BASF Corp. to achieve gas specifications for cryogenic processing and transportation via UK’s National Transmission System (NTS) to National Grid PLC’s gas network.
Subsequently held by Centrica PLC and now operated by Spirit Energy Ltd., the North Morecambe terminal has operated for 25 years despite changes to composition of feed gas delivered to the site. The success of the complex terminal since its commissioning results from a robust initial design and ongoing plant operation, detailed understanding of complex multicomponent adsorption processes, and the BASF adsorbent technology.
This article discusses the technologies and plant configuration supporting North Morecambe terminal’s gas processing operations and also presents a brief overview of new dehydration technology offerings BASF developed based on its collaboration at the North Morecambe site.
Gas treatment by adsorption
BASF’s hydrocarbon recovery unit (HRU) technology for the simultaneous removal of hydrocarbon and water (H2O) from natural gas is based on the properties of its proprietary Sorbead, a specialty alumino-silica gel adsorbent with a pore structure tailored to the adsorption of hydrocarbons such as C6+, benzene-toluene-xylene (BTX), and mercaptans. Sorbead adsorbent is manufactured in a unique oil-drop process that allows control over internal structure of the beads. The adsorbent’s homogenous structure, which eliminates particle-to-particle binding prevalent with other adsorbent products such as activated alumina or molecular sieves, is key to performance under adverse process conditions. This uniformity provides high crush-strength and attrition-resistant properties.
HRU operation uses multiple towers simultaneously, each in a different stage of the process. While one or more towers is in adsorption mode, one or two towers are in regeneration, which involves a first step of heating followed by a second cooling step. Feed gas is commonly used as regeneration gas that is then recycled to the tower in adsorption. Contaminates are removed as liquids from the system regeneration gas during cooling via a condenser, while the separated regeneration gas is recycled to the feed side of the adsorption unit. The short-cycle, dynamic-adsorption technology can be adjusted for specific adsorbent mass and cycle time so that the desired contaminant is removed.
While hydrocarbon removal technology requires short cycle times, adsorptive dehydration processes can achieve long-cycle operations. In the short-cycle HRU process, adsorbents are often cycled 10,000 times or more between changeouts, and the regeneration gas is a wet, heated feed- gas generating a hydrothermally severe environment during heating. The short-cycle process is more demanding on the adsorbent materials.
Terminal development study
Between 1985 and 1990, British Gas carried out a study to select the optimum process technology for a greenfield onshore gas processing terminal in Barrow-in-Furness designed to treat gas containing high concentrations of carbon dioxide (CO2), nitrogen (N2), hydrocarbons, and H2O produced from North Morecambe gas field development in the East Irish Sea off Northwest England, about 26 km west of Blackpool in water depts of around 20 m.
Based on gas composition, British Gas established upon inception that a portion of gas to be processed at the proposed North Morecambe terminal would need cryogenic processing for N2 removal to meet the NTS’ Wobbe index (WI) number (the ratio between calorific value and the square foot of gas density relative to air under ambient conditions) required for delivery into the national grid. Alongside a hydrocarbon dewpoint control system upstream of the cryogenic N2-removal process, the plant also would need an H2O-polishing dehydration unit between the hydrocarbon dewpoint control system and cryogenic section to reduce H2O dewpoint of gas to cryogenic specification since N2 removal requires cryogenic temperatures (OGJ, July 6, 2015, p. 100).
After evaluating capital and operational costs—and taking into account reliability and environmental considerations—British Gas selected BASF adsorption technology and adsorbent materials for design of the terminal’s H2O and hydrocarbon dewpoint control system (Sorbead HRU), its deep dehydration system (molecular sieve), as well as its two-trained CO2 and hydrogen sulfide (H2S) removal system (BASF OASE acid gas removal units, AGRU).1
Plant configuration
The heart of North Morecambe terminal’s gas conditioning process is the sequential two-stage adsorption design consisting of the HRU and molecular sieve dehydration unit that enable gas specifications required for cryogenic processing.
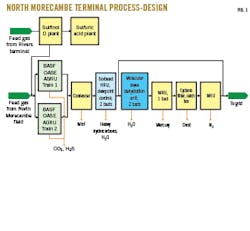
Fig. 1 shows the simplified process design of North Morecambe terminal’s main gas treatment train as commissioned in 1994.
After arriving at the terminal and passing through the OASE AGRU for removal of CO2 and other acid gas components, feed gas enters the HRU, which simultaneously removes any remaining heavy hydrocarbons and H2O in a single step. While a portion of this gas achieves sales specification and is sent directly to the grid, some volumes require cryogenic processing for N2 removal to meet the WI requirement. This gas moves to the downstream molecular sieve dehydration unit to reduce its H2O dewpoint to cryogenic specifications. Following water removal, the gas passes through the mercury removal unit (MRU) and N2-removal unit (NRU) before delivery to the grid.
This sequential adsorption-stage design has yielded many benefits for the plant, including extending the life of the molecular sieve dehydration bed to more than 15 years. During an adsorbent changeout in 2014, BASF and terminal operations staff concluded the molecular sieve—after 19 years in service—could have lasted much longer.2
HRU design
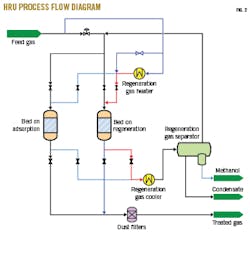
North Morecambe terminal’s Sorbead HRU is designed to remove 90% of H2O and selectively remove C7+ hydrocarbons from saturated gas simultaneously to achieve a cricondentherm (CHDP) of -10˚ C. The HRU achieves this CHDP—the highest temperature at any pressure at which a gas will begin to condense and hydrocarbon liquids form (e.g., maximum dewpoint)—using a two-tower unit, with one tower in adsorption and the other in thermal regeneration (Fig. 2). The regeneration gas is a fraction of feed gas taken from the adsorption gas, passed through a heater, and then sent downflow through the bed on regeneration. The hot, saturated gas is passed through a cooler-heat exchanger followed by a condensed liquids separator. Effluent regeneration gas is then reblended with adsorber feed gas to prevent loss of regeneration gas. After the adsorbent bed is heated to 270˚ C., incoming regeneration gas at ambient temperature is passed via a bypass around the heater to cool the Sorbead to ambient temperature in preparation for the next adsorption period on the freshly regenerated adsorbent. This process removes H2O to less than 30 ppmv, requiring the molecular sieve dehydration unit as an H2O polisher to meet the cryogenic NRU inlet specification.
Feed gas composition changes
Initially designed to process sulfurless gas from North Morecambe field, North Morecambe terminal still processes 100% of gas feedstock through its original Sorbead HRU unit. The terminal, however, also now treats gas production from a mixture of fields, including Spirit Energy’s South Morecambe and Rhyl fields, as well as increased volumes of sour gas (containing higher concentrations of H2S, CO2, N2, and mercaptans) produced at Chrysaor Resources (Irish Sea) Ltd.-owned Millom, Dalton, and Calder fields that it receives from Chrysaor Holdings Ltd.’s nearby Rivers terminal.
In addition to gas field composition variation, H2O content of feed gas also varies but is mostly independent of feed gas sources, as H2O content depends on temperature of gas leaving the amine CO2 removal unit.
Over the course of the Sorbead HRU’s operating lifetime, unit performance has been controlled by the adsorbent’s gradual decay in adsorption capacity. As a result, predictions of future adsorbent performance for changes in gas composition are generally based on current performance. Spirit Energy terminal operations staff regularly monitor current performance to assist BASF in subsequent performance predictions.
Methanol considerations
Methanol (MeOH), used as a hydrate inhibitor in gas processing operations, is also an impurity present in feed gas that affects North Morecambe terminal’s Sorbead HRU performance. Hydrate formation is a challenge, particularly in winter, when the air cooler is exposed to low ambient air temperatures. These conditions induce hydrate formation, causing blockages in the gas cooler. To reduce hydrate formation, terminal operations add MeOH to the stream in two locations, first at the sealine and again at the regeneration gas circuit. Because of high-H2O concentrations in hot gas at the cooler inlet during regeneration, MeOH is added into gas upstream of the cooler.
Addition of methanol in the gas treatment process results in excess MeOH in regeneration gas, and consequently, high-MeOH content in feed gas to the HRU. Since effluent regeneration gas is recycled to the inlet gas, the resulting adsorption inlet gas contains high, transient concentrations of gas-phase MeOH, which is subsequently readsorbed with H2O onto Sorbead adsorbent.
During an adsorption cycle, species that adsorb more strongly to the bed will displace other components that adsorb less strongly. The process is designed to switch to regeneration when C7+ hydrocarbons reach the end of the bed. At the end of the adsorption period, C7- hydrocarbons are about to break through while lighter hydrocarbons have been displaced and exited the bed. At the point of C7 breakthrough, C8+ hydrocarbons are fully adsorbed. Successful operation of the North Morecambe plant in meeting the required hydrocarbon dewpoint depends on the sufficient removal of C7+ components and initiating the regeneration cycle before breakthrough. Since MeOH shows a similar adsorption profile to H2O, it impacts available bed capacity for H2O and hydrocarbon adsorption.
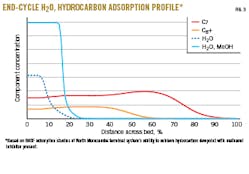
BASF and terminal operations performed theoretical studies to investigate the terminal system’s ability to achieve hydrocarbon dewpoint when MeOH is present. Fig. 3 presents results of these adsorption studies.
The studies considered an H2O and hydrocarbon adsorption profile in the bed at the end of the adsorption cycle. Solid lines represent a scenario with MeOH in the feed gas, while the dotted line shows the amount of the bed used when only H2O is present. Conclusions from this analysis suggest that, in the presence of MeOH (solid blue line), a larger portion of the top of the bed is used for adsorption, leaving less of the bed available for adsorption of C7 (red line) and C8+ (orange line) hydrocarbons. The initial plateau indicates the equilibrium zone, followed by the mass-transfer zone where adsorption takes place. H2O and MeOH are removed preferentially and adsorbed on the bed’s upper section. Hydrocarbon adsorption takes place in the lower, water-free zone. Due to presence of MeOH, there is a smaller portion of the bed available compared with an absence of MeOH (dotted blue line). This lower portion of the bed controls hydrocarbon dewpoint and overall performance of the adsorber unit. Depending on the level of MeOH in the feed, North Morecambe operations can adjust operations to maximize plant efficiency by extending or shortening adsorption times to achieve the same hydrocarbon dewpoint.
In addition to impacting bed adsorption capacity, excess MeOH in feed can lead to formation of dimethyl ether (DME). DME formation is the condensation reaction of two MeOH molecules (2 H3COH = H3COCH3 + H2O). This equilibrium reaction is driven to the right by continuous adsorptive removal of H2O on the bed, resulting in formation of more DME. This DME is removed from the bed during regeneration and is contained in the separated condensate, reducing the quality and value of condensate when reselling. The operation staff’s knowledge and understanding of MeOH’s impact on the adsorption process allows terminal operators to mitigate these effects and optimize plant performance.
Mercaptans removal
New feeds to the North Morcambe terminal exhibited the presence of various mercaptans that the Sorbead unit can remove along with heavy hydrocarbons and H2O.
Under certain conditions, however, tert butyl mercaptan (TBM) can react in the adsorption bed to form H2S. This increased concentration of H2S must be considered when designing a unit, and adsorption cycle times and regeneration settings must be decided accordingly.
In one instance when a new feed was delivered to the North Morecambe terminal, operators experienced spikes of H2S in the outlet stream. Upon investigation, operators discovered the new stream contained TBM, which was reacting to form H2S under regeneration. Once the team identified TBM in the stream and understood its concentration, gas treatment experts from North Morecambe and BASF cooperated to identify a solution.
Under atmospheric pressure, TBM, in the presence of a catalyst, breaks down at elevated temperatures of 330° C. With a pressure of about 75 barg during a regeneration and a temperature of only 270° C. on the bed inlet, some decomposition of TBM, and therefore formation of H2S, is still possible (2 C4H10SH = C8H18S + H2S).
The solution was to control the rate of temperature rise during regeneration, which kept H2S total concentration below the pipeline specification.
Operational performance, lifetime
Hydrocarbon dewpoint control is achieved by selective removal of C6+ hydrocarbons.
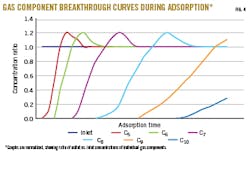
Fig. 4 shows a set of breakthrough curves of individual natural gas components during adsorption.
The normalized graphs show the ratio of the outlet divided by the inlet concentration. The outlet profiles show the typical increase of hydrocarbon concentration over time, rising from a low outlet value to a value above the inlet concentration. The displacement of adsorbed lower molecular-weight hydrocarbons by higher molecular-weight components causes this concentration ratio of > 1. Besides partial pressure of individual gas components, molecule polarity and boiling point are mechanisms controlling competitive hydrocarbon adsorption on a Sorbead bed.
Fig. 4 illustrates a displacement only based on hydrocarbon chain length; C5 is adsorbed first, then displaced by C6. The longer chain-length hydrocarbons are adsorbed sequentially and follow this same pattern. Hydrocarbon dewpoint control depends on the adsorption cycle time, which is switched to regeneration before breakthrough of C7+ hydrocarbons.
H2O adsorbs preferentially over most hydrocarbons and, as such, H2O concentration in feed gas impacts remaining available bed capacity for hydrocarbon adsorption to control dewpoint. The saturated water content of gas leaving the amine CO2-removal unit is controlled by the exit gas temperature and can reach 2,000 ppmv. The HRU’s duty is to adjust the H2O and hydrocarbon dewpoint to the required pipeline transport specification. BASF technologists and North Morecambe operations staff anticipated the HRU could achieve a much lower water dewpoint than required. The team executed a detailed on-site analysis to measure H2O outlet concentrations and discovered the treated gas only contained 15-20 ppmv of H2O.
The HRU reliably produced low H2O levels across the entire bed lifetime of 5+ years. Plant operations used this knowledge to adjust adsorption cycle times in the downstream molecular sieve dehydration unit. This adsorption unit is required to reach very low H2O concentrations (< 0.1 ppmv) necessary for liquefaction and nitrogen rejection. With an adsorption time of several days and a low-pressure regeneration system using dry nitrogen, the bed lifetime of the molecular sieve treater can exceed 15 years.
The effect of the upstream Sorbead HRU on the molecular sieve polisher’s bed performance cannot be overstated. By producing a very lean and dry gas, the risks of coking, retrograde condensation of hydrocarbons, or regeneration reflux in the downstream molecular sieve treater—all main causes of molecular sieve-bed aging—are essentially eliminated. The extremely long bed lifetime achieved at North Morecambe terminal is an important lesson learned that can be applied both to future plant designs and upgrades to existing operations.
Next steps
The North Morecambe terminal’s sequential adsorption-stage plant design points to implications for worldwide gas processing operations. Flexibility of the Sorbead HRU has enabled optimum utilization of the terminal as new gas compositions have been introduced, and long-term success of the combination of different adsorbents has given confidence to allow for developments in dehydration technology.
North Morecambe terminal’s sequential-adsorption unit design of an HRU followed by a dehydration unit serves as the conceptual basis for Durasorb Dehy units, which are designed to provide natural gas processors—particularly LNG plants—with more reliable dehydration service by combining Sorbead HRU know-how with molecular sieve materials. Sorbead HRU design software enables calculation of absorbent performance in all parts of the dehydration vessel, allowing BASF technologists to design a combination bed resistant to regeneration reflux and retrograde condensation. The combination-bed design increases performance and lifetime of adsorbent beds.
BASF has already completed installation of the technology in an African LNG plant, with results indicating the technology’s effectiveness in extending its dehydration unit’s life.3
References
- Mayer, M., and Crowe, T., “The North Morecambe Onshore Terminal,” GPA Europe Meeting: Production and Processing Difficult or Marginal Fields, London, Sept. 28, 1995.
- Eckardt, T., Wolf, E., Dolan, W.B., Wyatt, R., “Innovation in Materials Development for LNG Dehydration,” 19th International Conference on Liquified Natural Gas, Shanghai, Apr. 5, 2019.
- Greene, M., Pan, P., Eckardt, T., “Increased Dehydration Bed-Life Reduces Turnaround Costs and Eliminates Unplanned Shutdown: NGL and LNG Gas Plant Durasorb Case Studies,” BASF Corp., 2020. https://catalysts.basf.com/files/literature-library/Greene-BASF-Paper-2020.pdf
The authors
Martin Heywood ([email protected]) is engineering lead at Spirit Energy Ltd. in Barrow-in-Furness, Cumbria, UK, where he manages a multidisciplined team in the projects and engineering department. He joined Centrica PLC. (now Spirit Energy) in 2005 as a field process engineer at Barrow gas terminal in Barrow-in-Furness, UK. Heywood holds a BS (2000) with honors in chemical and process engineering from the University of Newcastle-upon-Tyne, UK. A Lean Six Sigma Green Belt, he is a chartered engineer and fellow of the Institution of Chemical Engineers, UK.
Matthew Uhe ([email protected]) is a principal process engineer at Spirit Energy Ltd. in Barrow-in-Furness, Cumbria, UK, where he provides technical oversight on operations and projects. After emigrating to the UK in 2013 following work as a process engineer in Australia’s energy industry, he joined Centrica PLC (now Spirit Energy) as an operations-focused process engineer supporting safe and efficient production activities. Uhe holds a BS (2009) with honors in chemical engineering from Monash University, Melbourne, Australia. A Lean Six Sigma Green Belt, he is a chartered engineer and member of the Institution of Chemical Engineers, UK.
Tobias Eckardt ([email protected]) is global technology manager for natural gas treatment and purification at BASF Catalysts Germany GMBH in Nienburg, Germany, where he also serves as key expert for natural gas applications on a global team of adsorption specialists. He previously held roles with the company in product development before gradually assuming increased managerial responsibilities. Eckardt holds a PhD (2005) in organic chemistry from the University of Cologne, Germany.
Margaret (Peg) Greene ([email protected]) is global market manager for adsorption solutions for natural gas at BASF Corp. in Iselin, NJ, where she works with her global team to bring new products to market and support BASF customers. After holding multiple positions throughout the company through the BASF leadership development program, she secured a role in the catalysts division as a technology manager before moving into her current role. Greene holds a PhD (2013) in chemistry from the University of California, Irvine.
Ray Racher is former global marketing manager for natural gas adsorbents at BASF Corp. in Baton Rouge, La., where he also previously served as global business manager for alumina and silica adsorbents. Before joining BASF in 2007, he held positions in technical and product management with Alcoa Corp. Racher holds a BSc (1977) in ceramic engineering from the University of Sheffield, UK. He is currently retired.
Roger Wyatt is a former senior consultant for BASF SE in Elmdon Heath, West Midlands, UK, where, as part of the adsorbents solutions group, he was involved in designing adsorption units for natural gas treatment. Before joining BASF, he worked on naphtha reforming pilot plant studies for more than 40 years at British Gas PLC’s Midlands Research Station. A chartered engineer in the UK, Wyatt holds a BSc (1965) in fuel science from Leeds University, UK. He retired in 2020.