MNOV: Developing SARS-CoV-2 Vaccine Based on Parainfluenza Virus Vector…
NASDAQ:MNOV
READ THE FULL MNOV RESEARCH REPORT
Business Update
Licenses SARS-CoV-2 Vaccine
On July 27, 2020, MediciNova, Inc. (NASDAQ:MNOV) announced the company entered into a joint development agreement with BioComo and Mie University for a SARS-CoV-2 vaccine using BC-PIV, a human parainfluenza virus (HPIV) type 2 vector. BioComo has initiated development of multiple vaccine candidates testing different stabilized Spike (S) protein mutants of SARS-CoV-2 in mouse models, and the agreement with MediciNova will enable additional preclinical work and GMP manufacturing to commence on a lead vaccine candidate such that clinical trials can initiate after an IND is filed with the FDA.
HPIV is an enveloped, negative-sense RNA virus that was first discovered in the 1950’s (Henrickson et al., 2003). There are four major subtypes of HPIV (denoted as types 1-4), with HPIV 1, 2, and 3 responsible for lower respiratory tract infections in children, the immunocompromised, and the elderly. HPIV 2 was selected as a vaccine vector as it does not integrate into the host genome and can cause repeated infections throughout an individual’s life due to incomplete immunity, thus there should be minimal risk for inactivation of an HPIV-based vaccine from an immune response even with repeated administrations, unlike with some adenovirus-based vaccines.
BC-PIV was developed using a reverse genetics system following removal of the F gene, which is required for viral cell-membrane fusion and viral proliferation (Hara et al., 2013). The virus is produced in a Vero cell line that expresses the F protein (Ohtsuka et al, 2014). The BC-PIV vector leads to very high expression of encoded transgenes (approximately 100-fold more efficiently than a conventional retroviral vector) and also displays the transgenic antigen on the viral envelope, similar to a virus-like particle (VLP) (Ohtsuka et al., 2019). Thus, BC-PIV can be administered without adjuvant, and due to its affinity for mucosal surfaces it will likely be possible to develop an intra-nasal formulation of the vaccine.
The ability for a BC-PIV vaccine to effectively induce neutralizing antibodies to a viral target was demonstrated with the Ebola virus. The Ebola glycoprotein (GP) gene was inserted into the BC-PIV genome and the vaccine was tested in an in vitro cell model of Ebola infectivity (Ohtsuka et al., 2019). The following figure shows the results of a neutralization test utilizing sera from mice immunized with PBS, BC-PIV/EBOV-GP, or BC-PIV/EGFP (enhanced green fluorescent protein, a marker of protein expression). The neutralization titers were similar to those seen with Ebola VLPs in mice (Takada et al., 2007).

FDA Approves Clinical Plan for MN-166 to Prevent ARDS Caused by COVID-19
On July 1, 2020, MediciNova announced that the company’s Investigational New Drug application (IND) for MN-166 (ibudilast) for the prevention of acute respiratory distress syndrome (ARDS) caused by COVID-19 was approved by the U.S. FDA and that a clinical trial may initiate.
The proposed trial will be a randomized, double blind, placebo controlled, parallel group study in hospitalized COVID-19 patients who are at risk of developing ARDS (NCT04429555). Eligible patients will be receiving standard-of-care therapy, which includes anticoagulation therapy. Inclusion criteria includes:
• a positive PCR test for SARS-CoV-2 infection
• chest imaging with abnormalities consistent with COVID-19 pneumonia
• SpO2 ≤ 92% on room air, respiratory rate ≥ 24 breaths per min, and/or requirement for supplemental oxygen
• At least one of the following risk factors: age > 65, underlying serious heart disease, chronic lung disease, moderate to severe asthma, BMI ≥ 40, or diabetes
Patients will be randomized to receive up to 100 mg/day MN-166 (50 mg b.i.d) or placebo for seven days, with follow-up examinations on days 14 and 28. The co-primary endpoints include the proportion of subjects free from respiratory failure (defined by the need for decreased oxygen requirements), the mean change from baseline in clinical status using the NIAID 8-point ordinal scale, the percentage of patients with improvement in clinical status, and the change from baseline in the following cytokine levels: macrophage migration inhibitory factor (MIF), interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and c-reactive protein (CRP).
MN-166 Identified as Potential Inhibitor of SARS-CoV-2 Replication
A recently published study screened 1,520 compounds from the Prestwick Chemical Library® to evaluate potential anti-SARS-CoV-2 activity in a SARS-CoV-2 virus infected cell-based assay (Touret et al., 2020). Less than 6% of the 1,520 compounds were identified as having anti-SARS-CoV-2 potency based on the in vitro screening for SARS-CoV-2 replication inhibition. Encouragingly, this study identified MN-166 (ibudilast) as a hit compound with potential anti-SARS-CoV-2 activity.
MN-166 Effective in ARDS Mouse Model
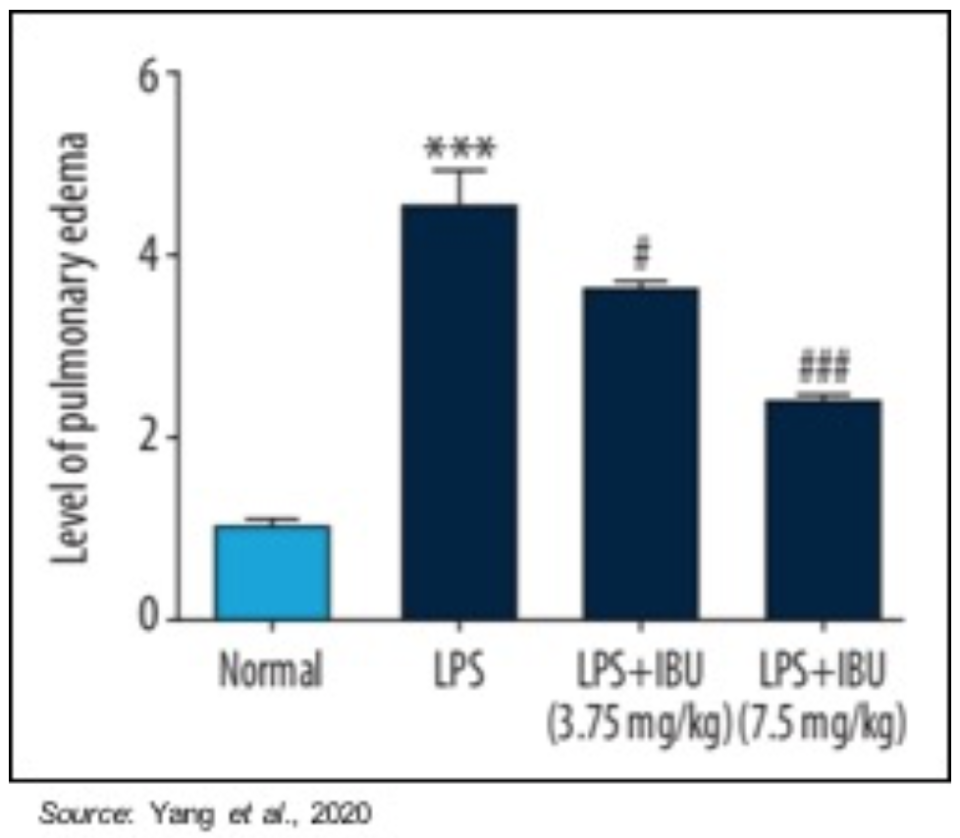
In a preclinical study, MN-166 was studied for its effectiveness on neonatal ARDS in a mouse model in which ARDS is induced with lipopolysaccharide (LPS) (Yang et al., 2020). Mice were divided into four groups of 10 each: a control group, a LPS-induced group, and two MN-166 treatment groups (3.75 and 7.5 mg/kg). The following figure shows that PDE4, which MN-166 is an inhibitor of, is increased by LPS stimulation and that treatment with MN-166 decreased this overexpression of PDE4 in lung tissue in ARDS mice.

In addition to decreasing the expression of PDE4, treatment with ibudilast also decreases the abnormal overexpression of different inflammatory cytokines, including TNF-a, IL-1b, IL-6, and MCP-1, and inflammatory chemokines, including CXCL1, CXCR4, and CXCR5.

Pulmonary edema was evaluated using the pulmonary edema score to indicate the amount of water accumulation in the lungs after pulmonary damage. Pulmonary edema was significantly reduced by MN-166 treatment (P<0.001). These results suggest that MN-166 may be able to reverse pulmonary edema, which is very important to the recovery of a patient suffering from ARDS.
The effect of MN-166 on lung cell apoptosis was also investigated. The following figure shows a TUNEL staining assay (which measures apoptosis; indicated by bright green) in which apoptosis (cell death) is prevalent in the untreated LPS sample, however the amount of apoptosis is decreased by MN-166 treatment, thus showing the drug’s ability to protect against pulmonary injury.

Inhibiting MIF May Improve Outcomes in Viral Infections
MIF is a protein that exhibits cytokine, endocrine, chaperone-like, and enzyme-like properties (Stosic-Grujicic et al., 2009). It binds to its high-affinity cell receptor CD74, leading to recruitment of CD44 and the mediation of a number of intracellular signaling pathways, including those involving the inflammatory cascade and the innate immune response (Calandra et al., 2003). MIF promotes the release of pro-inflammatory cytokines, such as TNF-α, IL-6, and prostaglandin E2. Elevated serum MIF concentrations are found in many infectious and inflammatory diseases. For example, MIF concentrations in sepsis patients correlate with disease severity (Bernhagen et al., 1993; Sprong et al., 2007) and anti-MIF antibodies protect mice in an in vivo model of septic shock (Calandra et al., 2000).
Numerous studies have shown the role of MIF in viral pathogenesis, thus potentially making MIF inhibition a suitable target for treating viral diseases, with a few of those discussed below:
• Arjona et al., 2007: This study investigated the role of MIF in the pathogenesis of West Nile Virus (WNV). The research showed that WNV patients had increased MIF levels in their plasma and cerebrospinal fluid. Blockade of MIF through three distinct mechanisms (antibody, small molecule, genetic deletion) increased resistance to WNV lethality in mouse models. The researchers concluded that MIF is involved in the pathogenesis of WNV and that targeting MIF could be useful in the treatment of WNV encephalitis.
• Assunção-Miranda et al., 2010: This study examined the involvement of MIF in dengue virus (DENV) infection and pathogenesis. Just as with WNV, patients with dengue hemorrhagic fever had elevated levels of MIF in their plasma and mif-deficient (Mif-/-) mice showed less severe disease following DENV infection, including a significant delay in lethality and lower viral loads compared to wild-type mice. These results again support inhibiting MIF as a therapeutic approach to treating DENV infection.
• Regis et al., 2010: This study investigated the role of MIF in patients with HIV-1 infection. Those with HIV-1 infection had elevated plasma levels of MIF and the HIV-1 protein gp120 induced MIF secretion from uninfected peripheral blood mononuclear cells (PMBCs). In addition, viral replication in PBMCs declined when the cells were treated with anti-MIF antibodies while viral replication was enhanced when recombinant MIF was added to HIV-1 infected PBMCs, thus showing that MIF is involved in promoting viral activity and inhibiting MIF could lead to decreased viral activity.
• Fox et al., 2018: The SPRINT-MS trial in patients with progressive multiple sclerosis (MS) showed that treatment with MN-166 resulted in a statistically significant decrease in the rate of decline in brain volume along with a 26% reduction in confirmed disability progression. In addition, an analysis of adverse events during the trial showed a statistically significant difference in upper respiratory tract infections, with only 10% of MN-166 treated patients reporting an upper respiratory tract infection compared to 19% of placebo treated patients (P=0.045).
The aforementioned studies show that MIF activity is related to viral pathogenesis, and viral replication, for a wide range of viruses and thus provides adequate support for using a MIF inhibitor in the treatment of viral diseases, including COVID-19 caused by the SARS-CoV-2 virus. The data from the SPRINT-MS trial is not related to any one particular virus, but is a real-world example of how MN-166 treatment can decrease overall viral infections, adding support to the notion that inhibiting MIF with MN-166 could be useful in treating viral infections.
Financial Update
On July 28, 2020, MediciNova filed form 10-Q with financial results for the second quarter of 2020. As expected, the company did not report any revenues for the second quarter of 2020. Net loss for the second quarter of 2020 was $4.5 million, or $0.10 per share, compared to a net loss of $3.9 million, or $0.09 per share in the second quarter of 2019. R&D expenses for the second quarter of 2020 were $2.2 million, compared to $1.5 million for the second quarter of 2019. The increase was primarily due to higher clinical trial expenses offset by lower stock-based compensation expenses. G&A expenses for the second quarter of 2020 were $2.3 million, compared to $2.7 million for the second quarter of 2019. The decrease was primarily due to lower stock-based compensation expenses.
Total operating cash burn for the second quarter of 2020 was approximately $2.1 million and the company exited the second quarter of 2020 with approximately $60.4 million in cash and cash equivalents. As of July 27, 2020, the company had approximately 44.4 million shares outstanding and, when factoring in the approximately 7.6 million stock options, a fully diluted share count of approximately 52.0 million.
Conclusion
We’re excited to see MediciNova enter the COVID-19 vaccine field and BC-PIV appears to be the only parainfluenza virus type 2 vector currently in development. HPIV has a number of positive attributes that may lead to the development of a successful vaccine against SARS-CoV-2, including the ability to express large amounts of antigen on its surface while maintaining its proper three-dimensional structure along with good intracellular expression. In addition, no adjuvant is required unlike many other COVID-19 vaccine candidates. Other potential advantages of the BC-PIV COVID-19 vaccine include intranasal delivery, which is convenient and can induce local mucosal immunity in addition to systemic antibody production, safety, and cost effectiveness. We look forward to updates on preclinical efficacy studies of the vaccine and additional information as to when clinical testing will begin. Our current valuation is $25 per share.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks provides and Zacks receives quarterly payments totaling a maximum fee of $40,000 annually for these services. Full Disclaimer HERE.
