Over the last few years, a developing field of research has emerged in breath gas analysis of volatile organic compounds (VOCs). This technique is non-invasive and has a number of possible applications, including monitoring of metabolic processes, study of pharmacokinetics, screening for disease biomarkers and drug testing.
Advantages of IONICON PTR-TOFMS
The detection limits and linearity range of IONICON PTR-MS systems are parallel to the concentrations of breath VOCs, making these devices highly effective tools for breath gas analysis. Furthermore, these systems can analyze breath online and in real time, thanks to the IONICON PTR-MS’s rapid response time and high sensitivity.
It takes just a fraction of a second for these devices to log the entire spectra, and in combining this speed with its high mass resolving power, the IONICON PTR TOFMS can detect multiple hundreds of compounds from just one exhalation.
BET-med Breath Sampling Inlet
For PTR-MS, IONICON has created a dedicated breath sampling inlet. This Buffered End-Tidal Breath sampler (BET-med) makes use of inert and heated surfaces to circumvent the risk of condensation, and can be utilized in conjunction with commercial, disposable mouthpieces.
Most significantly, it meets certification standards for medical use (ISO 60601) and thus enables real-time breath analysis in a clinical environment.
Screening for Breath Markers
The most typical application for this is in searching for breath biomarkers. In this type of screening study, comparisons are run between the breath spectra of subjects/patients with particular conditions and those of healthy volunteers. As the IONICON PTR-TOFMS instruments can analyze the entire spectrum at once, they are especially appropriate for this application [1].
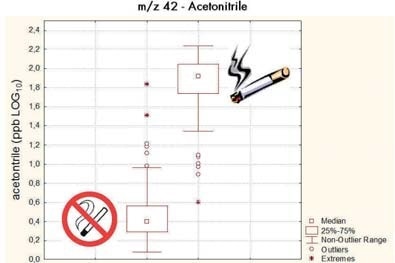
Figure 1. Analyzing the breath of more than 200 subjects, several markers for smoking can be isolated. Most prominently acetonitrile, which leads to an almost perfect separation of smokers and non-smokers.
The most conspicuous breath markers are those related to tobacco use, and they can thus be used as a benchmark for the technique. PTR-MS can reach a cross-validate accuracy (AUROC) of 99%, representing the greatest value published for a large study to this date [2].
Lung Cancer Markers
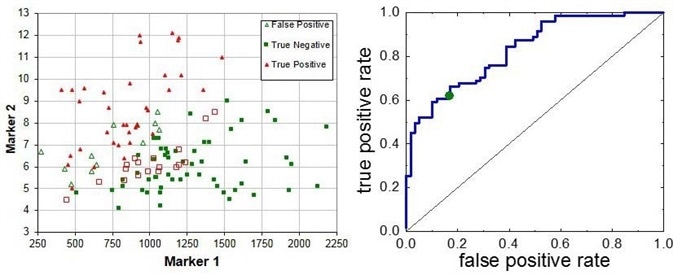
Figure 2. Breath markers for lung cancer require a careful analysis of the data. We found two robust markers that give an AUROC value of > 83% (cross-validated).
Biomarkers for lung cancer were able to be identified in a multi-centric clinical study. Through the combination of just two markers, it was possible to meet a cross-validated AUROC value of > 83% for the identification of bronchial adeno-carcinoma [3].
Monitoring Studies
The development of real-time breath analysis opened doors for a variety of new kinds of study in which a subject’s breath is monitored to track the fluctuations of one or more marker compounds over time. The subjects in these monitoring studies represent their own control, making data interpretation a much simpler task.
Pharmacokinetics
Pharmacokinetics is the name given to the study of the distribution and elimination of drugs in the body. Breath analysis allows for the non-invasive monitoring of the blood concentration of a drug, and offers data which is refreshed with each exhalation.
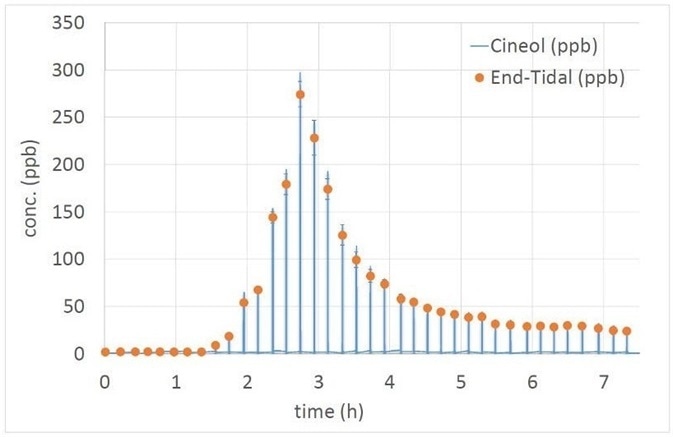
Figure 3. Pharmacokinetical study: full exhalations, recorded every 15 minutes, which depicts the concentration of a drug in the exhaled breath after ingestion (t=0).
In the figure, a sharp rise in the drug concentration is visible, as well as a less rapid decay, from which it is possible to derive pharmacokinetic models. For this behavior, a high sampling frequency is needed, something which would be close to impossible using blood tests or offline analysis [4].
Monitoring Metabolic Effects
The majority of volatile breath compounds have an impact on a number of metabolic processes. Through the distribution of isotopically labeled educts, the labeling of their metabolic products is also achieved, and these can thus be identified in a mass spectrum.
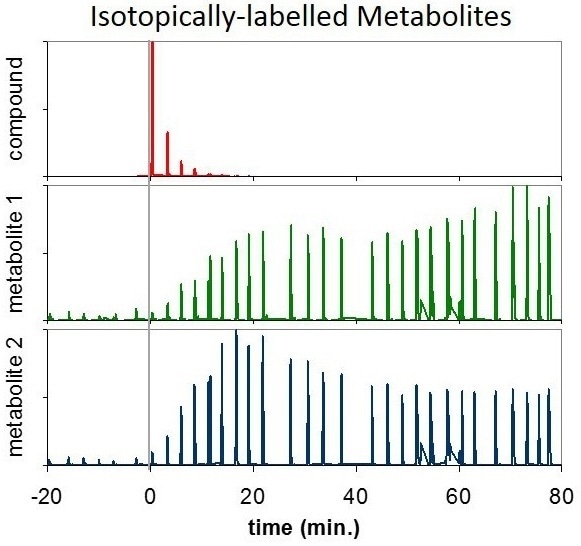
Figure 4. Two isotopically labelled metabolites (green, blue) with individual variations over time, which arise as a result of the ingestion of a labelled compound (red).
This enables researchers to investigate and study particular metabolic processes and deficiencies, making personalized medicine a distinct possibility for the future [5].
Sources
[1]: Herbig J, J Breath Res, vol. 3, no. 2, IOP, pp. 027004 (2009)
[2]: Herbig J, 4th Int. Conf. on PTR-MS, IUP Conf. Series, pp. 46-50 (2009)
[3]: Herbig J, 5th Int. Conf. on PTR-MS, IUP Conf. Series, pp. 31-33 (2011)
[4]: Beauchamp J, J Breath Res, vol. 4, no. 2, IOP, pp. 026006 (2010)
[5]: Winkler K, J Breath Res, vol. 7, no. 3, IOP, pp. 036006 (2013)
About Ionicon Analytik Ges.m.b.H.
IONICON Analytik was founded 1998 as a spin-off company of the University of Innsbruck, Austria commercializing an innovative technology called Proton Transfer Reaction - Mass Spectrometry.
Since then they have been improving this leading-edge technology resulting in the development of several types of ultra sensitive high resolution online mass spectrometers for monitoring and quantification of volatile organic compounds (VOCs) in sub-pptv level concentrations.
Today they are the world’s leading producer of trace gas analyzers with market-leading, real-time, single-digit pptv-level detection limits using the unique Proton Transfer Reaction – Mass Spectrometry (PTR-MS) and Selective Reagent Ionization – Mass Spectrometry (SRI-MS) technology.
Their product portfolio includes quadrupole MS and time of flight MS based instruments, industrial VOC monitoring solutions and custom built instrumentation, complemented by their own range of trace calibration devices and accessories.
Since 1998, they are serving their customers in many different application areas including atmospheric chemistry, environmental research and trace gas analysis (e.g. emissions in urban and remote areas, indoor spaces, vehicles), food and flavor science (e.g. analysis of coffee, olive oil, butter, cheese, wine, herbal extracts and aromas), industrial VOC monitoring (e.g. in waste incineration plants, chemical factories and production sites of the semiconductor industry) and illicit substances detection (Explosives, CWAs, TICs).
A special focus was given to medical and biotechnological applications where real-time monitoring of industrial fermentation processes, of synthetic gas production processes in the petrochemical industry and real-time breath gas analysis in clinical settings are carried out.
They also offer analytical services and are an active contributor to numerous international scientific research and training projects.
In 2016 they proudly celebrated 300 PTR-MS instruments sold. They manufacture their precision instruments at their company site located in Innsbruck, Austria.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.