Stress
Stress, Inflammation, and Microbes: A Moody Trinity
Psychological stress leads to physical stress, anxiety, and depression.
Posted November 30, 2019 Reviewed by Gary Drevitch

Everyone knows that our moods are controlled by our minds. That's common knowledge—but it's not completely true.
Our moods are strongly influenced by hormones as well, something we are familiar with, but tend to forget. Our moods are also affected by our immune system and—strangest of all—our gut microbes. To add to the complexity, pressures in our lives run roughshod over this tangled biological network.
Stress is how the body responds to the pressures of life. It works by trying to bring things back into balance, pulling on the strings of immunity, hormones, and nerves. Stress, from your boss or a bad oyster, may lead to depression and anxiety. In either case, it can all start with the immune system.
The Immune System Remembers
The immune system knows to hit the panic button as soon as the pathogens attack. But the immune system can also respond to psychological stress—so-called sterile stress. Fighting invaders is one thing, but steeling us against mental pressures seems like a big ask of the immune system.
There is a surprisingly simple way to demonstrate the close association between stress and immunity: Introduce an ordinary mouse to a bully mouse for a few minutes a day. It only takes a week or two for the intimidated mouse to develop inflammation as measured by pro-inflammatory chemicals in their blood. Among other things, their lymphocytes—white blood cells—undergo a change.
Mice specially bred to have low levels of lymphocytes are anxious and less sociable than typical mice. But something interesting happens when you inject these shy mice with lymphocytes from the bullied mice: Contrary to what you might expect, they become less anxious and start to socialize more.
In the stressed mouse, these lymphocytes were trying like crazy to reduce the inflammation induced by the bully. This is one of nature's most enduring circuits: the negative feedback loop, working to prevent runaway reactions. The psychological stress of harassment primed those lymphocytes to suppress inflammation. Once transferred, they exerted a beneficial "eustress" effect on the host mouse, causing a reduction in pro-inflammatory chemicals and conferring resiliency on their new host.
This interesting experiment shows that purely psychological stress can make changes to the immune system that are remembered and can be transferred to another mouse, altering its behavior. This suggests a possible treatment for depression by simply rebalancing the immune system.
What About Therapy?
So, if inflammation is at the heart of stress-related depression, how does therapy work? Oddly enough, treatments like cognitive behavioral therapy can actually lower the levels of pro-inflammatory chemicals.
The development of antidepressants has led to a great revolution in psychiatry, but how do they work? One of the first theories was that they "topped off" neurotransmitters in the brain. Serotonin, it was thought, was depleted in people with depression, so any drug that could increase its level in the brain was going to be beneficial. Selective serotonin reuptake inhibitors (SSRIs), which keep serotonin circulating in the brain, do exactly that. But the mechanism may need some reinterpretation in light of the fact that SSRIs also reduce inflammation in the brain.
The immune system is deliriously complex, and we understand very little of it, but recent research makes it clear that it is tightly bound up with depression, and that reducing inflammation can reduce depression.
How Do Microbes Fit In?
We've seen how repeated stress can lead to an immune response and how the resulting inflammation can lead to depression. But what role do microbes play?
The world is liberally carpeted by bacteria, many of which would like nothing better than to eat us for breakfast. In response to this nasty reality, animals long ago recruited their own set of friendlier microbes to protect themselves. These beneficial bacteria are not really on our side so much as they are simply happy to ferment away in our warm and wet intestines, consuming a continuous buffet. These bacteria are called commensal, Latin for "sharing a table."
When pathogens try to intrude on this long-established partnership, commensals tear them to pieces. Our microbiota, in essence, is the vanguard of immunity. In order to establish such an important relationship with human hosts, commensals need to teach the immune system to tolerate them. This happens early in life, before a thousand days have passed, and if the process is disturbed, that person may be plagued by a lifelong proclivity to illness.
Like sheepdogs guiding a flock, immune cells monitor commensals to keep them corralled in the gut and out of blood circulation. The bacteria, for their part, secrete fatty acids that pacify immune cells, protecting them from attack. Through these secretions, gut bacteria can exert their effects far from the gut, and even change the shape and function of the brain.
Amazingly, that means microbes can alter your disposition. Those that improve your mood, including certain species of Lactobacillus and Bifidobacteria, even reduce cortisol levels. These are called psychobiotics, and they represent another possible treatment for depression. They have been shown to reduce negative thoughts, improve cognition, and lower symptoms of IBS, although these are early days for the field.
Microbes can affect the size of your hippocampus or amygdala, brain centers that are involved with mood, appetite, and fear. The hippocampus tries to keep the HPA axis from going off the rails, and if its growth is stunted, that affects the stress reaction. A balanced immune system is achieved by dialing everything down to a low simmer. A slightly elevated immune reactivity is sufficient for the brain to sample the microbiota and other stressors, but not enough to boil over into a major inflammation.
Conducting the Orchestra
If immune cells and microbes are the key players in this biological orchestra, the HPA axis is the conductor. Short for hypothalamus-pituitary-adrenal, the HPA axis coordinates the release of hormones that build in a crescendo to the release of cortisol, the drug responsible for the fight-or-flight response.
Cortisol is the body's way of amping up the stress response by increasing blood pressure, heart rate, and breathing in order to jump from a snake or run from a badger. In the process, it also dials down the immune response: First, you escape the badger, and only then do you deal with your flu or your food poisoning. That's a reasonable response for a badger encounter, which is only a few moments of panic. But repeated over time, that lowered defense can lead to a leaky gut, converting psychological stress into microbial stress.
The HPA axis communicates with your microbiota and your brain, using several different chemicals:
- Bacterial secretions: Metabolites like short-chain fatty acids.
- Immune chemicals: Cytokines like interferons, interleukins, and tumor necrosis factors.
- Glandular secretions: Hormones like adrenaline and cortisol.
- Nerve transmission chemicals: Neurotransmitters like serotonin, dopamine, and GABA.
Your brain communicates directly with your gut through the vagus nerve, and microbes can talk back on the same channel. The baroque complexity of this network provides several different opportunities to tweak the immune reaction. Consult the figure below to see how the main components interact.
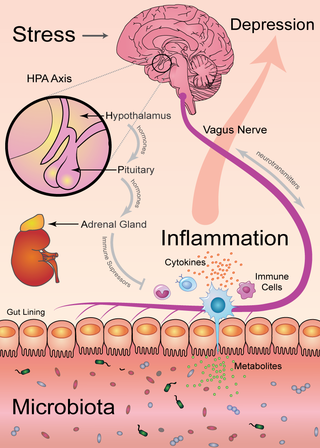
The microbiota is a central player in this concerto, and it can be changed by diet. That means you can intervene in the stress-depression axis. With the right diet and possibly some supplements, you can improve the composition of your microbiota and thus increase your resilience to stress.
- Eat fiber. Fiber has been stripped from processed foods in order to make them delicious, but many of your more helpful microbes depend on fiber. The real problem with junk food is that it starves your beneficial microbes. Fiber can be found in onions, artichokes, asparagus, and many leafy greens. The Mediterranean diet emphasizes these foods and is a great model to follow.
- Avoid antibiotics. These are life-saving drugs, but they have the nasty side effect of killing your gut microbes. Use prudently.
- Try prebiotics. If you can't get enough fiber in your diet, prebiotics offer a similar effect. These are complex sugars that your body can't digest, but your gut microbes can.
- Exercise. Oddly, sitting on your butt is not good for your gut. If exercise were a drug, it would be worth millions. It's not clear exactly how it works, but moving your body improves the quality of your microbiota.
- Sync your schedule with daylight. Your microbes have circadian rhythms, just like you. If those two rhythms are synced with each other, life is syncopated goodness. Get up with the sun, and your microbes will thank you.
Depression can come from grief, loss, or just a lousy boss. But it can also be caused or exacerbated by your microbiota. That is hard to wrap our heads around, but it means that we all have a chance to gain the upper hand. Be nice to your gut bugs, and you will find your mood improving. Your mileage may vary, but wouldn't it be silly not to give it a try?
References
Brachman, Rebecca A., Michael L. Lehmann, Dragan Maric, and Miles Herkenham. “Lymphocytes from Chronically Stressed Mice Confer Antidepressant-Like Effects to Naive Mice.” The Journal of Neuroscience 35, no. 4 (January 28, 2015): 1530–38.
Pereira, Joana da Cruz, Kieran Rea, Yvonne M. Nolan, Olivia F. O’Leary, Timothy G. Dinan, and John F. Cryan. “Depression’s Unholy Trinity: Dysregulated Stress, Immunity, and the Microbiome.” Annual Review of Psychology 71, no. 1 (2020)
Del Grande da Silva, Giovanna, Carolina David Wiener, Luana Porto Barbosa, Jaciana Marlova Gonçalves Araujo, Mariane Lopez Molina, Pedro San Martin, Jean Pierre Oses, Karen Jansen, Luciano Dias de Mattos Souza, and Ricardo Azevedo da Silva. “Pro-Inflammatory Cytokines and Psychotherapy in Depression: Results from a Randomized Clinical Trial.” Journal of Psychiatric Research 75 (April 2016): 57–64.
Pan, Ying, Xu-Yang Chen, Qing-Yu Zhang, and Ling-Dong Kong. “Microglial NLRP3 Inflammasome Activation Mediates IL-1β-Related Inflammation in Prefrontal Cortex of Depressive Rats.” Brain, Behavior, and Immunity 41 (October 2014): 90–100.
Jacka, Felice N., Adrienne O’Neil, Rachelle Opie, Catherine Itsiopoulos, Sue Cotton, Mohammedreza Mohebbi, David Castle, et al. “A Randomised Controlled Trial of Dietary Improvement for Adults with Major Depression (the ‘SMILES’ Trial).” BMC Medicine 15, no. 1 (January 30, 2017): 23.




