Gilead's Rheumatoid Arthritis Drug Successful in Phase III
Gilead Sciences, Inc. GILD and partner Galapagos NV GLPG announced that the phase III FINCH 2 study on rheumatoid arthritis candidate, filgotinib, was successful.
Filgotinib, an investigational, selective JAK1 inhibitor met all primary and key secondary endpoints in first phase III study in rheumatoid arthritis.
The global, randomized, placebo-controlled, phase III study evaluated filgotinib in in adults with moderately-to-severely active rheumatoid arthritis and prior inadequate response/intolerance to biologic agents. The trial achieved its primary endpoint in the proportion of patients achieving an American College of Rheumatology 20% response (ACR20) at week 12. Both the doses — 100 mg and 200 mg — achieved significantly higher ACR20/50/70 responses than placebo in patients with active rheumatoid arthritis and prior inadequate response to biologic agents.
However, two cases of uncomplicated herpes zoster were reported in each filgotinib group.
The details of the study will be presented at a future conference.
We remind investors that Galapagos and Gilead entered into a global collaboration for the development and commercialization of filgotinib in inflammatory indications in 2016.
Along with FINCH 1 and 3, the phase III FINCH 2 trial is one of the several clinical trials of filgotinib in rheumatoid arthritis. Other trials include the phase II EQUATOR program in psoriatic arthritis, the TORTUGA study in ankylosing spondylitis, the phase III DIVERSITY trial in Crohn`s disease (also small bowel and fistulizing Crohn`s disease phase II studies) and the phase III SELECTION trial in ulcerative colitis.
Last week, both the companies had announced positive results from the randomized, placebo-controlled phase II study, TORTUGA, on filgotinib. The trial is evaluating the safety and efficacy of filgotinib in adult patients suffering from moderately-to-severely active ankylosing spondylitis (AS).
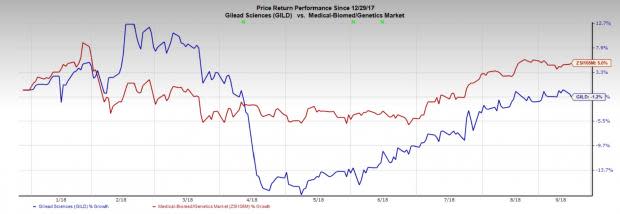
Meanwhile, the successful development and commercialization of the candidate will boost growth for Gilead. Gilead’s stock has lost 1.2% in the year so far, lagging the industry's gain of 5%.
Given the persistent decline in HCV sales, the company is looking to HIV and newer avenues to boost its top line.
Gilead is intending to foray into the non-alcoholic steatohepatitis (NASH) and inflammation market with late-stage candidates, selonsertib and filgotinib, respectively.
During the first quarter of 2018, Gilead announced an agreement with Sangamo Therapeutics, Inc. SGMO to use Sangamo’s zinc finger nuclease technology platform for the development of next-generation ex vivo cell therapies in oncology.
However, Gilead will have to generate substantial revenues from its HIV franchise and Yescarta to offset the HCV sales decline. This will be a challenging task for the company with stiff competition from the likes of GlaxoSmithKline GSK in the HIV market.
Zacks Rank
Gilead currently carries a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Wall Street’s Next Amazon
Zacks EVP Kevin Matras believes this familiar stock has only just begun its climb to become one of the greatest investments of all time. It’s a once-in-a-generation opportunity to invest in pure genius.
Click for details >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
Gilead Sciences, Inc. (GILD) : Free Stock Analysis Report
Sangamo Therapeutics, Inc. (SGMO) : Free Stock Analysis Report
Galapagos NV (GLPG) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

