February 21, 2017
The National Academy of Sciences has become the first major institution to endorse the use of gene-editing technology in human embryos to prevent the birth of children with serious diseases.
Kristopher Sturgis
|
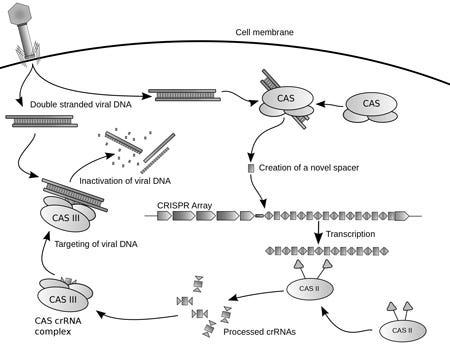
A diagram of the CRISPR prokaryotic viral defense mechanism. |
The National Academy of Sciences, one of the nation's most influential science advisory groups, announced its support for the use of CRISPR to modify human embryos in a series of heritable germline genome editing trials. The endorsement came last week in a 216-page report that was researched and written by a 22-member panel of leading scientists and experts.
The advisory group made it very clear that their endorsement extends only to alterations that are designed to prevent children from acquiring genes that are known to cause serious diseases and disabilities, and only when there are no other "reasonable alternatives." The report also noted that the technique must be approached with extreme caution and oversight.
"Heritable germline genome editing trials must be approached with caution," the report said. "But caution does not mean that they must be prohibited."
Despite the numerous potential benefits that the technology offers, this kind of human gene editing has been fraught with ethical caveats that could pose significant threats to the exploitation of the technology. Researchers fear that the technology could eventually be used to enhance intelligence or physical attributes to create a superior human race, and although the panel believes it will be years before the technique is safe enough to consider for regular use, they believe science should only proceed with the technique to prevent disease.
CRISPR has been a hot topic of debate for some time now as researchers continue to explore the technology and refine its editing abilities. This past summer a U.S. federal biosafety and ethics committee unanimously approved the world's first human study using the CRISPR technology to create genetically modified T cells that could target and destroy tumor cells. With each new study, researchers hope to forge a path toward editing out specific genetic defects and diseases from the human genome, as well as providing a new tool to fight disease.
This new report could be a significant step in that direction, as new trials could eventually lead to a form of germ line engineering that could create a future where people can have children without passing on the genes for diseases like Huntington's, Tay-Sachs, and other fatal genetic conditions.
However, as it stands, germ-line modification is prohibited in the U.S. ever since pro-life legislators barred the idea in a U.S. Department of Health and Human Services appropriations bill in 2015. The bill forbids FDA from even considering any proposal to create genetically modified offspring--meaning any proposal to modify human embryos to create a child will be ignored. The legislation will be up for periodic renewal, upon which the possibility for a path to clinical use could be opened up.
As research moves forward, the panel outlined in their report that the line between avoiding serious disease and human enhancement may eventually prove to be a blurry one. Controlling the technology could be the most difficult aspect of moving forward with the technique, as there are very real concerns that doctors and scientists will go overseas to attempt to use the technology where there is little or no regulation.
For now the panel makes it clear that their endorsement for the use of the gene editing technology comes without the consideration of political ramifications--something the panel considers beyond their expertise. The panel says that public discussions must precede any decision to pursue clinical trials, and this panel's recommendation could be the first step towards a more serious conversation.
Kristopher Sturgis is a contributor to Qmed.
[Image courtesy of Wikipedia]
About the Author(s)
You May Also Like