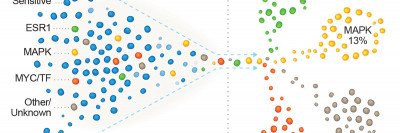
Most breast cancers are ER-positive, meaning the cancer cells have a receptor that enables estrogen to fuel their growth. Breast cancer therapies aim to block this process, but the cancer cells often develop resistance.
Most breast cancers depend on the hormone estrogen to fuel their growth. They are defined as estrogen receptor positive (ER-positive) and account for about 70% of cases diagnosed. Cancer cells in these tumors bear a receptor (ER) — a protein — that becomes activated when estrogen binds to it.
People with ER-positive breast cancer can be treated with drugs called aromatase inhibitors, which prevent production of estrogen and starve the cancer of this fuel. But for many of these patients, aromatase inhibitors stop working as the cancer spreads elsewhere in the body.
Now a Memorial Sloan Kettering research team has discovered how mutations in a gene called FOXA1 are causing this resistance to aromatase inhibitors in some people. The researchers identified two groups of mutations, which occur in different regions of the FOXA1 gene. The mutational groups, called Wing2 and SY242CS, appear to cause the cancer cells to grow and resist aromatase inhibitors in distinct ways.
“We are seeing two mutational groups that drive this resistance using different mechanisms,” says Amaia Arruabarrena-Aristorena, the study’s first author. “Understanding the details of how the resistance develops is very important because it raises the possibility that alternative hormone therapies might be effective in these patients.” Dr. Arruabarrena-Aristorena is a research fellow in the laboratory of MSK cancer biologist Maurizio Scaltriti.
She points to existing drugs such as fulvestrant (Faslodex®), which binds to ER receptors so they stop working. Although it is too early to say for sure, people whose tumors have FOXA1 mutations may avoid aromatase inhibitor therapy and benefit from such alternative treatments.
The study, published September 3 in Cancer Cell, was supervised by Dr. Scaltriti and Eneda Toska, a senior research scientist in Dr. Scaltriti’s laboratory in the Department of Pathology and Human Oncology and Pathogenesis Program and incoming assistant professor at Johns Hopkins University.
The researchers made the discovery with the help of physician-scientist Pedram Razavi, who analyzed the genetic information of samples taken from about 5,000 patients. In parallel, they conducted lab experiments with cancer cells, both in a lab dish and implanted in mice. The findings need to be confirmed in more patients, but they could potentially improve treatment for a large proportion of people with breast cancer.
A Noteworthy Protein
Dr. Scaltriti says his team first became interested in studying FOXA1 in 2018. A study led by Dr. Razavi provided a hint that FOXA1 mutations were associated with resistance to hormone therapies. Dr. Razavi had performed genetic sequencing of more than 1,900 breast tumors using MSK-IMPACTTM, which can detect mutations and other critical changes in the genes of both rare and common cancers.
“With such a large database of patients, it makes it possible to pick up on even small phenomena,” Dr. Scaltriti says. “That paper was a starting point from which many projects stemmed out, including this study of FOXA1 mutations.”

Some of the study co-authors (from left): Eneda Toska, Srushti Kittane, Amaia Arruabarrena-Aristorena, and Maurizio Scaltriti.
FOXA1 already stood out in other ways. The protein produced by this gene, called FOXA1, is a transcription factor. These proteins turn genes on or off by binding to chromatin, the complex of DNA and proteins that make up chromosomes. Tightly packed chromatin must be opened before genes can be turned on.
Typical transcription factors cannot open the chromatin alone. FOXA1, however, is a pioneer transcription factor — it can pry open chromatin by itself. This opens the floodgates, allowing other transcription factors and co-activators to access the DNA and turn genes on.
“It was exciting for us to study FOXA1 — not only because of its possible connection to therapy resistance but also because of its unique function as a pioneer factor and, through the investigation of cancer-associating mutations, we are learning more about its pioneering role,” Dr. Toska says.
To gain a clearer understanding of how FOXA1 mutations cause resistance, the researchers looked at areas within the FOXA1 gene in which mutations were especially prevalent in breast cancer patients. In the lab, they genetically manipulated breast cancer cells to see how they behaved in an environment of estrogen deprivation to mimic the effect of aromatase inhibitors.
Contrasting Effects
By studying breast cancer cells with selected mutations at different spots within the FOXA1 gene, the team was able to learn which mutations most caused resistance. Cells with Wing2 or SY242CS mutations were less affected by estrogen deprivation and grew more. But the researchers found there was a striking difference in how the mutations cause resistance.
The SY242CS mutation alters the FOXA1 protein shape so that it binds to and opens new sites of the chromatin. This enables FOXA1 to turn new genes on or off to regulate their expression, which, it turns out, gives the cells the capacity to grow more even in the face of estrogen deprivation.
Wing2 mutations, on the other hand, seem to amp up the cells’ ability to respond to the estrogen that is available. This allows the cell to grow even when estrogen levels are limited, similar to a tenacious plant that can survive with scarce nutrients.
The next step is to study the mechanisms of both mutational types further. Dr. Razavi is expanding the genetic analysis to include more patients with breast cancer, which should add clarity to the effects of these mutations and provide more evidence to guide treatment decisions.
“If a doctor sees a patient with a FOXA1 mutation, maybe they should lean toward a therapy like fulvestrant, but we need to validate this in a bigger group of patients,” Dr. Toska says.
“Only in institutions with this unique access to so many samples and a clear commitment to translational science are these kinds of findings possible,” Dr. Scaltriti says.