Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biotechnology
Young blood may hold the weapons for targeting age-related diseases
Young blood isn’t a fountain of youth, but some of its molecular factors could help regenerate neurons and blood vessels, researchers say
by Melissa Pandika, special to C&EN
October 13, 2019
| A version of this story appeared in
Volume 97, Issue 40

Around 15 years ago, reports began to emerge that taking blood from young mice and giving it to old mice seemed to dial back the clock on aging for the elderly rodents. The older mice showed signs of regenerating brain, muscle, and other tissues and boosting cognitive and physical performance. These findings came on the heels of other experiments that resurrected a rather macabre technique known as parabiosis, which dates to the mid-1800s. Like something out of science fiction, parabiosis is a procedure in which researchers suture together young and old mice so that they share a circulatory system.
Scientists initially used parabiosis to investigate how conjoined organisms, like some twins, affect each other. Specifically, they wanted to know whether molecules in the bodily fluids of one could alter the physiology of the other. After a period of declining interest in the method starting in the 1970s, parabiosis returned to the scene in 2005, when Stanford University scientists decided to use the approach to answer questions about tissue regeneration in older organisms. Aging is marked by an impaired ability in most tissues to regenerate themselves; the researchers performed parabiosis in mice to tease apart whether this was due to intrinsic changes in cells or some change in their surroundings.
After the release of the 2005 study and other work showing that young blood could seemingly rejuvenate old mice, scientists and the public alike seized on the alluring notion of an elixir of youth. In 2017, the Monterey, California–based start-up Ambrosia began selling transfusions of young plasma—the liquid component of blood—for $8,000 per liter, despite the lack of clinical trials showing this treatment was safe and effective in humans. But excitement was soon tempered by fears of a dystopian future involving the mandatory harvesting of blood from the young to sustain the old, although bioethics experts dismissed this scenario as the stuff of science fiction.
In February, concerned about premature application in humans based on findings in mice, the US Food and Drug Administration cautioned against young plasma transfusions, noting that they have “no proven clinical benefit” for age-related or other diseases in humans. The same day the FDA released its statement, Ambrosia announced it would stop its transfusions. Now, the start-up has completely closed shop, Business Insider reports.
In the wake of the initial fervor surrounding young blood, researchers are taking a more measured approach. Rather than trying to reverse aging, they’re identifying the molecular factors responsible for the changes seen in parabiosis experiments in hopes of targeting specific diseases associated with aging, such as age-related macular degeneration or Alzheimer’s disease. “Right now, conducting a clinical trial for aging is extremely difficult,” says Eric Verdin, CEO of the Buck Institute for Research on Aging. A targeted approach could yield practical treatments more quickly and with fewer ethical and other concerns than simply transfusing patients with young blood, researchers say.
Brain boosters
The relative abundance of aging and regenerative factors in our bodies shifts as we age, Verdin explains. At birth, our blood contains more regenerative factors—like oxytocin, which has been shown to rejuvenate skeletal muscle stem cells in mice—than aging factors (Nat. Commun. 2014, DOI: 10.1038/ncomms5082). But as we get older, that balance gradually tips in favor of aging factors, like the protein eotaxin, thought to play a role in age-related diseases in which systemic inflammation occurs. Aging factors lower our ability to maintain and repair tissue structure and function, while regenerative factors raise it.
Parabiosis experiments in mice give researchers a way to identify regenerative factors: researchers can compare the levels of various proteins in the blood of old mice before and after they receive blood from their young partners. The procedure is relatively straightforward, certainly less technically challenging than giving mice blood transfusions, researchers say. The factors that show a significant increase after parabiosis may be responsible for any observed rejuvenating effects in mice.
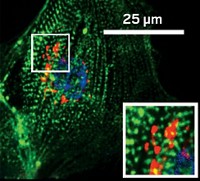
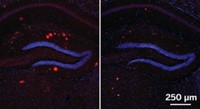
Among the many biological functions that decline with age is neurogenesis, or the growth of new neurons. Some researchers, like those in neuroscientist Saul A. Villeda’s lab at the University of California, San Francisco, hope parabiosis experiments could help identify the factors that promote neuronal growth. Those molecular factors might lead the way to treatments for age-related cognitive decline or other neurological diseases of aging. Because of stem cells’ role in new tissue growth, Villeda and his team looked for stem cell–related factors whose levels rose in old mice after parabiosis. They first scanned hippocampal neurons, ones in the brain’s center for learning and memory. The only one that significantly increased was Tet2, an enzyme that chemically tags genes with a hydroxymethyl group, altering their expression. These tagged genes, the team discovered, play a role in the growth of neurons (Cell Rep. 2018, DOI: 10.1016/j.celrep.2018.02.001).

The researchers found that Tet2 levels in the hippocampi of mice—and the chemical tags added by the enzyme—decreased with age but increased in mice that had undergone parabiosis. What’s more, when the researchers blocked Tet2 activity in the hippocampi of young adult mice, neuronal growth decreased, and the animals performed worse on memory and learning tests. When the researchers overexpressed Tet2 in mature adult mice, neurogenesis returned to the levels seen in younger mice, and the animals’ memory improved. Now, Villeda and his team are determining which types of neurons Tet2 acts on, which could, in turn, reveal the types of memory the enzyme affects.
Meanwhile, molecular and cellular physiologist Thomas C. Südhof of Stanford University and Kathlyn Gan, a postdoc in his lab, are identifying molecules that promote the formation of synapses, structures that allow neurons to communicate with neighboring neurons. Synapse function diminishes with age. The duo saw more synapses form between neurons grown in young blood serum than those grown in old blood serum. Two proteins—THBS4 and SPARCL1—appeared at higher levels in young serum (Proc. Natl. Acad. Sci. U.S.A. 2019, DOI: 10.1073/pnas.1902672116). Adding them to cultured neurons in a petri dish boosted synaptic formation. Südhof and Gan now want to pinpoint the receptors on the surface of neurons that bind THBS4 and SPARCL1 and investigate whether boosting synapse formation might counteract the loss of synapses that likely occurs early on in neurodegeneration. In mice, at least, regenerative factors show promise in restoring neuron growth and cognitive function to more youthful levels.

Burgeoning blood vessels
Other research teams are focused on what young blood can reveal about blood vessel formation. Abnormalities in the formation of blood vessels are seen in Alzheimer’s disease and numerous other diseases of aging. Alkahest, a biotech company in San Carlos, California, has invested in studying eotaxin, which increases in our bodies with age. In particular, Alkahest is interested in the role eotaxin plays in age-related macular degeneration (AMD). In AMD, abnormal blood vessels grow in a layer of tissue beneath the retina called the choroid, seeping fluid or bleeding into the retina and causing blindness; eotaxin promotes this blood vessel formation when it binds to a receptor located on these blood vessels.
To reduce abnormal choroidal blood vessel growth, Alkahest developed a molecule, AKST4290, that prevents eotaxin from binding to the receptor. The firm recently reported Phase IIa clinical trial results showing that AKST4290 improved visual acuity in patients with previously untreated AMD. AKST4290, an oral medication, could be “really impactful,” says Elizabeth Jeffords, chief commercial and strategy officer at Alkahest. That’s because clinicians typically treat AMD by injecting a standard drug therapy into the eye, a much riskier and more invasive delivery method.
Researchers at another biotech company, Elevian, are betting on a protein called GDF11 to tackle age-related disease. Like AKST4290, GDF11 is involved in blood vessel growth.
Elevian founders verified that GDF11 increased in old mice that had undergone parabiosis. Researchers at the firm then injected old mice with GDF11 and found that they experienced blood vessel regrowth throughout the brain (Science 2014, DOI: 10.1126/science.1251141), as well as neuronal growth in regions of the brain’s subventricular and subgranular zones (Sci. Rep. 2018, DOI: 10.1038/s41598-018-35716-6). The researchers hypothesize that GDF11 spurs neurogenesis by coaxing the cells lining blood vessels to secrete factors that trigger neuron formation. In other words, GDF11 seems to work not only by increasing neurogenesis but also by enhancing the brain’s blood supply, Elevian cofounder Lee Rubin says.
But not everyone is on board with this hypothesis. Efforts to replicate Elevian’s findings suggest that GDF11 may in fact impair neurogenesis. For instance, a 2018 study conducted by researchers at the Chinese Academy of Medical Sciences and Peking Union Medical College found that GDF11 promoted programmed cell death and maturation in neural stem cells and prevented them from migrating, which they need to do to repair damaged tissue (PeerJ 2018, DOI: 10.7717/peerj.5524). Consistent with Elevian’s findings, though, researchers at Jiaotong University, Fudan University, and other institutions reported that GDF11 rejuvenated the brain’s vasculature in mice (J. Alzheimer’s Dis. 2018, DOI: 10.3233/JAD-170474). Despite conflicting results
, Elevian still believes GDF11 holds promise, and the company continues to search for suitable applications for it as well as ways to extend its half-life, says Elevian CEO and cofounder Mark Allen.
A more complex picture emerges
Other researchers who study aging think that young blood could point us to more than just regenerative factors for treating disease. Research to slow or halt aging is more complex than searching for regenerative factors in blood, says Irina M. Conboy of the University of California, Berkeley. The 2005 study on the rejuvenating effects of parabiosis, done by Conboy and her colleagues while she was a postdoc at Stanford, helped catapult interest in the therapeutic potential of young blood (Nature 2005, DOI: 10.1038/nature03260). She points out, though, that in parabiosis, animals share not only a circulatory system but also their immune and organ systems, making it difficult to rule out these systems’ influence on aging or rejuvenation.
A decade after that study, to control for effects from these other bodily systems, Conboy’s lab developed a parabiosis-like technique that allows mice to exchange only blood. When the researchers used the technique to connect young and old mice, they found that after each mouse had equal parts old blood and young blood circulating through it, the young mouse displayed negative effects. Old blood drastically decreased hippocampal neuron generation, learning and agility, and liver regeneration in young mice (Nat. Commun.2016, DOI: 10.1038/ncomms13363). Young blood, on the other hand, showed no significant benefits for cognition, agility, or the generation of hippocampal neurons in old mice. In other words, the secret to stalling aging may not lie in boosting rejuvenating factors but instead in blocking factors in old blood that promote aging—ones that hinder tissue maintenance and repair.
Conboy and team have just begun to identify these aging-related factors. Recently, they’ve turned their focus to TGF-β, a protein that increases with age. In a study published in August, they showed that pharmacologically normalizing the activity of the TGF-β pathway, which is elevated in old age, while adding the rejuvenating factor oxytocin improves muscle regeneration, enhances hippocampal neuron growth, and boosts cognition, among other effects, in old mice (Aging 2019, DOI: 10.18632/aging.102148). But Conboy predicts that staving off aging will require normalizing multiple factors. “You cannot write or play a symphony with one instrument,” she says. “No one molecule will stop aging.”
Advertisement
The Buck Institute’s Verdin doesn’t see combination therapies emerging for a while, though. For now, researchers are still trying to identify the individual factors involved. Plus, it’s much easier to get FDA approval for single factors than combination therapies.
Treatments that deliver individual factors targeting specific conditions may feel more palatable than dystopian scenarios of forced blood donation by the young, but they still raise thorny ethical questions. Most likely only the wealthy would have access to these factors, which are not likely to be covered by insurance or Medicare and Medicaid, says Craig M. Klugman, a bioethicist at DePaul University. This, in turn, could result in a healthier, longer-lived wealthy class and shorter-lived middle- and lower-income classes. Klugman also notes that improving the lives of the majority by devoting resources to their basic needs—which we can already do—“outweighs the science fiction dreams of a few.”
On the other hand, aging, with all the chronic diseases it causes, also “takes an incredible toll on society,” Verdin says. He doesn’t see the quest to make people live healthier, longer lives as that much different from the medical advances over the past century that have shifted the average life expectancy. “Someone could make the argument, ‘You’re living long enough,’ ” he says. But not Verdin; he sees what many now accept as the end point of life as “the start of the human adventure.” The slow, rigorous search for molecules that restore our ability to maintain and repair our tissues—though perhaps less flashy than a young-blood elixir—may make this adventure possible.

Melissa Pandika is a freelance writer. A version of this story first appeared in ACS Central Science: cenm.ag/youngblood.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter