The current economic climate has forced hospitals to scrutinize their spending in an effort to reduce costs and increase revenue. As a result, many hospitals are focusing their supply spending in areas that will help them save money. With Medicare no longer reimbursing healthcare facilities for the costs of treating certain preventable infections, one area of focus is infection prevention.
August 6, 2010
Hospitals are increasingly investing their time, effort, and dollars in comprehensive infection control strategies to protect patients and bottom lines.
This presents an opportunity for medical device manufacturers, which are also feeling the pinch from the economic downturn. With hospitals willing to invest funds in products to combat hospital-acquired infections (HAIs), manufacturers are finding that they can differentiate themselves and meet the market need through devices that incorporate a silver-based antimicrobial. As a result, hospitals are looking to integrate antimicrobial devices, such as needleless connectors and pain management catheters, into their overall infection control plans. This article presents detailed information on silver-based antimicrobials and how they work, background on the use of silver-based antimicrobials in medical products, and insights from infection control advocates on the use of FDA-approved silver-protected devices to promote cleaner, safer healthcare environments.
The Fight Against HAIs
In many ways, hospitals are fighting an uphill battle against HAIs, which account for an estimated 1.7 million infections and 99,000 deaths each year.1 Researchers estimate that the direct medical costs of HAIs to U.S. hospitals is anywhere from $28.4 billion to $45 billion annually.2
Legislative, biologic, and economic factors place great pressure on hospitals to control the HAI epidemic. In 2008, Medicare ceased reimbursing healthcare facilities for the increased costs of treating certain preventable infections.3 And these infections are costly—the U.S. Department of Health & Human Services reported that healthcare costs for treating Staphylococcus aureus bloodstream infections for Medicare patients exceeded $2.5 billion in 2005.1
Compounding this problem is the rise in antibiotic-resistant organisms or superbugs, including Methicillin-resistant Staphylococcus aureus (MRSA). MRSA kills an estimated 20,000 people in the United States each year and a recent study found that infections have risen by 90% since 1999.4
The Role of Medical Devices in HAIs
There are four categories of infections that account for three-quarters of the HAIs that occur in acute care hospitals, as follows:
?Surgical site infections.
?Central line-associated bloodstream infections.
?Ventilator-associated pneumonia.
?Catheter-associated urinary tract infections.
Research has shown that medical devices used on or within the body are a significant source of HAIs. According to the Multidisciplinary Alliance Against Device-Related Infections (MADRI), at least one-half of all HAI cases are medical device associated. This is primarily due to the fact that medical devices placed on damaged skin or inserted into the body provide an ideal environment for disease-causing organisms.
Read how antimicrobials |
Unlike the human body, device polymers do not have an immune system to fight off bacteria. As a result, bacteria colonize the surfaces of these devices, multiply, and develop a biofilm, which is an organized community of bacteria protected by a slime layer.
According to Marcia Ryder, PhD, MS, RN, of Ryder Science, a medical biofilm research facility in San Marcos, CA, when microorganisms unite to create a biofilm on a medical device, they undergo cellular changes that make them resistant to antibiotic treatment.
“Antibiotics are developed and tested against microorganisms living in a planktonic or free-floating growth phase,” Ryder says. “When bacteria attach to surfaces, they begin to communicate, cooperate, and build a structured community; they up-regulate and down-regulate genes. They become profoundly changed and different from that bacteria floating around in a broth so the antibiotics that we order to kill them have limited effect.”
For more than 20 years, Ryder has been researching medical biofilms, focusing on the role that medical device biofilms play in HAIs. She points out that all medical devices are prone to biofilm formation because any surface that we place on or within the body will grow bacteria.
“This is a huge problem for the healthcare community and we’re not doing a good job at addressing it,” Ryder says. “Think about HAIs and the sources of these infections—urinary catheters, central venous catheters, endotracheal tubes. [HAIs] are all medical device [related] biofilm diseases. And because we are far from being able to effectively treat these infections, prevention is key.”
Giving Devices a Defense Mechanism
To address this deficiency, manufacturers are protecting devices against microbes in various ways. One particular method is through silver-based antimicrobials. This is so that the devices most associated with high rates of infection have their own defense mechanisms and provide hospitals with an additional weapon in their arsenal against HAIs.
Silver-based antimicrobial-treated devices are not meant to replace so called active hygiene practices (e.g., hand washing and the use of disinfectants) but serve to supplement them. Because the silver inhibits biofilm formation continuously with no human intervention required, these devices provide a passive means of reducing microbial contamination.
“It’s foolish to say that we are just going to try harder to get doctors and nurses to clean their hands and follow proper procedures,” says Betsy McCaughey, PhD, founder of the Committee to Reduce Infection Deaths (www.hospitalinfection.org) and former lieutenant governor of New York. “If we have the technology to prevent these infections then it is immoral not to use it. Antibiotic or silver-treated devices are like airbags or antilock disc brakes. They serve as safety measures that back up good intentions and compensate for human fallibility. Furthermore, we can demonstrate that they are highly cost effective.”
As HAIs become a critical issue for hospitals across the globe, a growing number of medical device manufacturers have developed products that feature silver-based antimicrobials. These can be used in a variety of products such as urinary catheters to reduce the risk of urinary tract infections and in endotracheal tubes to ward off ventilator-associated pneumonia. From the manufacturer’s perspective, silver-treated devices provide a competitive edge in today’s challenging healthcare marketplace.
Vygon, an international medical device company headquartered in Ecouen, France, has developed a complete range of adult single- and multilumen central venous catheters (CVCs) with silver-based antimicrobial protection, as well as a silver-protected adult peripherally inserted central catheter (PICC).
“Today, everyone is looking for a product with an antimicrobial,” says Alan Martin, director of training and education for Vygon. “That’s given us major advantages over our competition because we can offer a product with antimicrobial protection without a major premium.” Clinical studies conducted with Vygon’s Multicath Expert catheters have shown that they reduce bacterial contamination and subsequent bloodstream infections.
A developer of infusion therapy technology, Medegen Inc., has also produced a line of silver-based antimicrobial-protected needleless intravenous flow valves. The MaxGuard advanced luer activated device helps prevent contamination and growth of microorganisms at the point of entry into the catheter and subsequently into the bloodstream.
Why Silver?
The idea of using antimicrobial-treated devices often raises a red flag in the minds of both healthcare professionals and medical device manufacturers. Is the antimicrobial safe? Will it contribute to the development of antibiotic-resistant superbugs? Does it work? How much cost does it add to the manufacturing process?
Not all antimicrobials are created equal. Manufacturers certainly don’t want to use therapeutic antibiotics to treat medical devices because antibiotics contribute to resistant organisms or are ineffective against them. Safety is also a primary concern because the antimicrobial is released onto or into the patient’s body over a period of time. In addition, the antimicrobial must be effective against a broad range of organisms. Finally, it must be durable enough to be processed into the device material. Ionic silver satisfies these requirements in various applications.
Safety. Known as nature’s antimicrobial, silver is less toxic than table salt and less irritating than talcum powder. A naturally occurring element, silver has long been used as an antimicrobial. As early as 4000 BC, the Egyptians would line their water vessels with silver when traveling on long journeys to keep microbes from spoiling the water. In more recent times, it has been widely used in wound and burn care.
|
Figure 1. Silver works through three modes of efficacy. It suffocates (inhibits) the membrane transport process, sterilizes (prevents cell multiplication), and starves (interrupts cell metabolism). |
Resistance. Therapeutic antibiotics typically feature one mode of attack against microbes so it takes just one mutation for the microbe to become resistant to the drug. But silver fights microbes in three ways, reducing the chance of resistance (see Figure 1).
Efficacy. Ionic silver has been proven effective against nearly all of the pathogens of concern in healthcare environments, including Staphylococcus aureus, Escherichia coli, Methicillin-resistant Staphylococcus aureus, Staphylococcus epidermidis, Klebsiella pneumoniae, Pseudomonas aeruginosa and Candida albicans.
In one recent study, researchers analyzed Staphylococcus epidermidis colonization on standard CVCs versus silver-treated CVCs that had been inserted in patients during renal transplant procedures. Among the standard CVCs, 24 out of 41 (58.5%) exhibited bacterial growth while only one of the silver-treated catheters (6.6%) had a biofilm.
Furthermore, none of the silver-treated catheters had to be removed due to local infection or intolerance. The researchers concluded that the silver-treated catheters led to a marked reduction in catheter-associated infections of the bloodstream.5
Another study published in the November 2008 issue of Pediatric Neurosurgery found ventricular catheters impregnated with silver were more effective in inhibiting bacterial colonization (Staphylococcus aureus, Staphylococcus epidermidis and Pseudomonas aeruginosa) compared with antibiotic-impregnated and standard catheters. The researchers concluded that silver-treated catheters were more advantageous for the prevention of catheter contamination and subsequent shunt infections.6
Silver in the Manufacturing Process
One of the challenges of using organic compounds, such as antimicrobial drugs, disinfectants, and sanitizers, in medical devices is that they do not have the stability required to be processed into the device material. As a result, they must be applied as a coating to the device after it has been manufactured. Antimicrobial coatings add expense to the manufacturing process, plus it can be challenging to ensure that the coating will stay on the device for the length of time required to be effective.
|
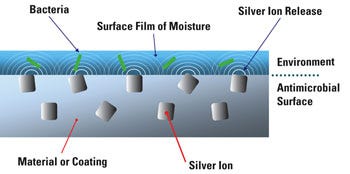
Figure 2. Silver ion technology works through a release of ions through the surface of the polymer. |
Silver, an inorganic, particulate antimicrobial, is durable enough to be processed into the device material. The manufacturer is not required to add an additional step and there are no concerns that the antimicrobial will detach itself from the surface of the polymer. As a result, the cost of silver antimicrobial has minimal impact on the overall manufacturing cost (see Figure 2).
The Importance of Controlled Release
Although all silver-based technologies employ the same active ingredient—ionic silver—how the silver is delivered greatly influences its effectiveness against microbes. The challenge is to have a design strategy that delivers the silver gradually over time in a difficult environment (i.e., on the body, in the body, and in the blood) for the desired antimicrobial lifetime.
The two methods of silver delivery for medical devices are dissolution and controlled release. Dissolution describes a silver metal or compound that simply dissolves. This would be a silver-coated device that, once implanted, provides a burst of silver ions. A controlled-release mechanism, on the other hand, facilitates the gradual release of silver over a specified period of time.
|
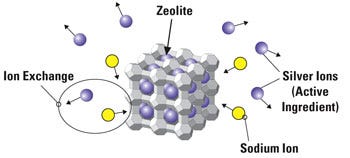
Figure 3. The zeolite cage holds silver ions, thereby enabling a controlled release. |
Depending on the medical device and its purposes, it is likely that a controlled-release strategy is more effective against microbes. Two common cases are a urology catheter that requires antimicrobial protection for a period of 7 days, a CVC for 49 days, and a permanently implanted device that must combat microbes for a period of years. These are, without question, devices that benefit from a silver-based antimicrobial that features a controlled release mechanism. Control release for these devices enables the manufacturer to generate a device that provides optimal performance for the defined lifetime of the product (see Figure 3).
FDA’s Stance on Antimicrobials
As medical device manufacturers have increasingly incorporated silver and other antimicrobials into their devices, FDA has evolved its position on the regulatory approval process for these products. In 2007, FDA issued a draft guidance to assist the industry in preparing and submitting 510(k)s for medical devices that feature antimicrobials. This 18-page document outlines the specific information that FDA requires with respect to such products. FDA requires not only information related to the design and function of the device, but also the various characteristics of the antimicrobial technology.
Although manufacturers know the ins and outs of their devices, they are typically not antimicrobial experts. For most, FDA’s requirements related to the antimicrobial agents can be complex and confusing. To facilitate successful and timely delivery to market, device manufacturers should choose an antimicrobial technology partner that can guide them through FDA’s device evaluations and providing the necessary information at critical points in the process (see the sidebar “Antimicrobial Agents: FDA Requirements”).
In addition to providing information to help the manufacturer answer technical questions related to the antimicrobial, the technology partner should also have the capabilities to design and perform the experiments required to generate supporting data, interpret these data, and complete the concluding calculations to satisfy the FDA’s requirements. Furthermore, the technology partner should provide guidance to the manufacturer on regulatory submissions for explaining the antimicrobial and its relevance to the device.
Silver-Protected Devices Today
As hospitals confront greater liability and revenue loss resulting from HAIs, they are increasingly adopting antimicrobial-treated medical devices as part of their overall infection control strategies.
According to McCaughey, there has been greater awareness and interest in silver-treated medical devices among clinicians in recent years and patients are becoming advocates of the technology as well. In her opinion, educating hospital administrators on how these products can improve their bottom line is critical to widespread adoption. She says that nurse have shown particular interest in the silver-infused products. The challenge is that manufacturers must demonstrate to administrators that by purchasing these devices they can reduce overall costs, increase revenue, and reduce the risk of litigation.
Ryder highlights the opportunity for both manufacturers and providers in the healthcare industry and stresses the importance of clinical research in the development of antimicrobial-treated devices. “The development of new technology utilizing antimicrobial coatings and biomaterial surface modification presents both financial opportunity for industry and, importantly, the opportunity to impact the lives of many by prevention of costly and deadly healthcare-associated infections,” Ryder says.
Ryder’s advice to manufacturers in the development of new technology is to gain a solid understanding of biofilm-related infections, work with medical biofilm researchers to design clinically simulated models for in vitro, in vivo, and clinical research, and understand the capability of the product at each level of testing. They should also make modifications to enhance effectiveness. Finally, she says, “do not go to market without sound efficacy data—clinicians should and will demand scientifically rigorous data to accomplish the demand for evidence-based practice.”
Conclusion
Factors have converged to make this a crucial time for the healthcare industry to reduce the incidence of HAIs. As hospitals and other healthcare facilities seek new ways to improve the cleanliness of their environments without relying solely on human intervention, manufacturers are meeting the need with silver-based antimicrobial-treated devices that can be used to supplement and enhance existing infection control strategies.
The current focus on infection prevention presents a significant opportunity for the medical device industry, but simply adding an antimicrobial to a device is just not enough. When developing an antimicrobial-treated medical device, manufacturers must take into consideration a wide range of factors that are critical to the product’s success, including the safety and efficacy of the antimicrobial of choice, as well as the regulatory path required for approval. To do this, they must work hand in hand with their antimicrobial technology partner, regulatory agency, and the broader healthcare community to deliver a product that is safe, effective, and marketable.
References
1.HHS Action Plan to Prevent Healthcare-Associated Infections: Executive Summary, U.S. Department of Health & Human Services; available from Internet: www.hhs.gov/ophs/initiatives/hai/infection.html.
2.R Douglas Scott II, “The Direct Medical Costs of Healthcare Associated Infections in U.S. Hospitals and the Benefits of Prevention,” (CDC) March 2009; available from Internet: www.cdc.gov/ncidod/dhqp/pdf/Scott_CostPaper.pdf.
3.Centers for Medicare & Medicaid Services; available from Internet: www.cms.gov/HospitalAcqCond/.
4."Drug-Resistant Bacteria on the Increase in U.S.," Reuters Health, November 24, 2009; available from Internet: www.reuters.com/article/idUSTRE5AN0N020091124.
5.J Loertzer et al., “Use of Catheters with the Agion Antimicrobial System in Kidney Transplant Recipients to Reduce Infection Risk,” Transplant Proceedings 38 (2006): 707–710.
6.HI Secer et al., “Comparison of the Efficacies of Antibiotic-Impregnated and Silver-Impregnated Ventricular Catheters on the Prevention of Infections,” Pediatric Neurosurgery 44 (2008): 444–447.
Jeffrey A. Trogolo is chief technical officer for Agion Technologies (Wakefield, MA).
About the Author(s)
You May Also Like