Chemical Strategies for Assembling the Bifunctional Surface
There are a number of examples of bifunctional MNPs found in the literature and the strategies can be vaguely categorized into the followings. Figure 7 illustrates the differences between these approaches.
Figure 7.
Four strategies for constructing the multifunctional surface on a magnetic nanoparticle.
(A) Multifunctionality on a polymer chain. (B) Use of block copolymers. (C) Cocondensation of alkoxysilanes. (D) Secondary reaction on grafted groups.
MNP: Magnetic nanoparticle.
Building on the Polymer Skeleton
As discussed previously, not all polymers used as the first coating layers have active organic groups for constructing a multifunctional surface. We shall focus on those polymers applicable to such designs, namely dextrans, chitosan, PVA and PEI.
Dextrans are polysaccharides of glucose units with α-1,6 glycosidic linkages between them (Figure 3A & 3B). In terms of chemistry, the only active groups on a dextran molecule are the hydroxyl groups (-OH), three on each glucose unit except the branching units. Due to these -OH groups, dextrans are very hydrophilic and water soluble. Dextrans have already been used in many biomedical applications (e.g., as an anticoagulant)[55] and therefore it is a popular choice for coating MNPs.[36] Although the -OH groups are quite stable at room conditions, activation of these groups can be achieved using some chlorinated organic compounds or epoxides. For example, carboxylic acid can be attached onto the dextran using chloroacetic acid (Figure 3B), which is also a commercial method to manufacture carboxymethyl cellulose (CMC).[56] Also, such property allows crosslinking of dextran using epicholorhydrin (Figure 3A). These functionalization strategies can also be used to functionalize PVA, which is a synthetic, nonbiodegradable polymer with a -CH2CH(OH)- repeating units, and the -OH groups have similar chemical properties to those on dextrans. As such, a multifunctional surface may be synthesized by using two or more chlorinated organic reactants in the grafting reaction.
Several polymers have a readily bifunctionalized structure for biomedicines. For example, chitosan is a family of polysaccharide molecules with an amine group (-NH2) replacing one of the -OH groups on the glucose ring (Figure 4). Unlike -OH groups, -NH2 groups are ready to bind many biomolecules (Table 1). Indeed, many binding protocols, including EDC/NHS coupling, for protein binding are based on the chemistry of the amine groups on the protein molecules. Chitosan is also highly biocompatible and has been widely used in tissue engineering.[57,58] Due to these amine groups on the polymer chain, chitosan has also been used for DNA binding and transfection, but only with a rather low efficiency recorded for the unmodified polymer.[59] Chitosan can also bind negatively charged drugs (including the nonsteroid anti-inflammatory drug diclofenac and gadopentetic acid for cancer treatment) and act as a delivery agent.[60] Due to the coexistence of the -NH2 and -OH groups in the structure, chitosans can be employed to build a multifunctional surface. However, many reagents used to activate -OH groups (e.g., chloroacetic acid) also react with -NH2 groups (Table 1), hence it is necessary to block the -NH2 groups first in order to avoid deactivation. One possible solution is to use a chitosan precursor, chitin, instead as chitin is an acylated form of chitosan and there is no free -NH2 available for reaction (Figure 4B). After activation of the -OH groups on chitin, deacylation can then be carried out to 'free' the -NH2 groups, usually using a base. Subsequently, a bifunctional chitosan surface can then be synthesized.
Another widely used positively charged polymer is polyethyleneimine (PEI),[61] which has a repeat unit of -CH2CHNH- (secondary amine) and a terminal -NH2 (primary amine) groups. Since there are some contrasts in chemical properties between primary and secondary amine groups, multiple functional groups can be built on PEI polymers. For example, aldehydes only react with primary amines to form imines but not secondary or tertiary amines. This chemical property can be selectively used to activate the terminal -NH2 groups while the secondary amine groups are preserved, and therefore results in a bifunctional polymer surface.
Use of Functional Block Copolymers
Although it is possible to build a multifunctional surface by grafting multiple functional groups on a polymer skeleton, it can also be built by coupling polymer chains of different functionalities together (Figure 7B). This design is commonly termed as block copolymers. The major advantage of using block copolymers is that specific functional groups can be built separately before joining together. As such, interference in chemistry is to be minimized during grafting of various groups.
Such a block copolymer design has been demonstrated as early as 2002. Burke et al. used a poly(styrene-block-tetraethylenepentamine) (PS-b-TEPA) to coat MNPs (30–40 nm).[62] However, this design was orientated to the synthesis of MNPs rather than having any biomedical applications in mind. This is because, in the early development, efforts had been focused on the stabilization of MNPs using polymers. Nonetheless, this provides a good example of block copolymer-coated MNPs and, since then, research on using block copolymers for coating MNPs has started to grow. For example, poly(vinyl alcohol-block-vinyl amine) was used to coat MNPs.[45] Whilst the PVA was to provide biocompatibility, the amine groups on the polyvinyl amine block allowed the tagging of a fluorophore, Cy3.5, for tracking purposes. The results also showed that such a synthetic block copolymer has a low toxicity effect on cells.
One popular choice of block copolymer is poly(lactic acid-block-ethylene glycol) (PLA-b-PEG). Both PLA and PEG have been widely used in biomedical research and proved to be nontoxic. In addition, the amphiphilic structure (part hydrophobic, part hydrophilic) of PLA-b-PEG block copolymer allows self-assembly of micelles, which are stable in water and physiological environment. This property of PLA-b-PEG was exploited by Nasongkla et al. when RGD-tagged PLA-b-PEG micelles were assembled for the delivery of doxorubicin, an anticancer agent.[63] An MRI contrasting property was also introduced by adding hydrophobic MNPs (8 nm in diameter, oleic acid coated) into these micelles. A detailed study on the colloidal stability of a similar system was also published recently by Bakandritsos et al..[64] However, since there is no direct chemical bonding between the MNP core and the copolymers, these systems are only stabilized as micelles, which can be broken down if the environment changes significantly (e.g., heat or extreme pH). Furthermore, the MNP-loaded, RGD-tagged micelles may not be as nontoxic as suggested from this report because these micelles seemed to have triggered a 20% cell death in 4 h when compared with the control experiments (no micelle loading).[63]
After these early research works, the block copolymer systems became more complex, including an increase in the number of blocks. A research group in the USA led by Misra has reported the use of a triblock copolymer dextran-g-poly(NIPAAm-co-DMAAm) for coating MNPs.[65] Poly(NIPAAm) is known to be thermal sensitive with a lower critical solution temperature (LCST; the critical temperature when the polymer collapses and releases all the trapped molecules, e.g., drugs) of 32°C in water. In order to become useful in vivo (body temperature 37°C), the LCST of the polymer has to be increased to a temperature near 37°C. As such, a small temperature change in the body or the environment can initiate the change in the polymer structure. The addition of poly(DMAAm) block of a specific length helped to raise the LCST to 38°C, which is just above the human body temperature. The goal of such design is to build MNPs with a drug (such as doxorubicin) carrying capacity and releasing them under a thermal control.[66] Thermal release can be triggered by either the temperature difference in different parts of the body or by magnetic hyperthermia, which is a unique phenomenon of MNPs and is currently being studied as a new therapy for cancers.[67]
Recently, a magnetic emulsion system was developed based on the use of triblock copolymers. Woodward et al. added bromofunctionalized MNPs into the emulsion and the shape of these magnetic emulsion changes in the presence of a magnetic field.[68] Magnetic sorting of these magnetic emulsions was also demonstrated. However, the real benefits of these magnetic emulsions in biomedical sciences are yet to be discovered.
Cocondensation Silanization
We have previously introduced the use of alkoxysilanes, with a wide variety of functional groups, for the first coat of MNPs. Since the chemistry of grafting these groups onto the MNPs surface is rather similar, using mixed alkoxysilanes is possible for creating a multifunctional surface (Figure 7C). However, a number of points regarding their chemical properties have to be considered for choosing the right mixture:
The organic groups may react with each other; for example, APTES will react with bromopropyl ethoxysilane due to the reaction between -NH2 and -Br groups (Table 2);
There are differences between the rates of silanization according to the organic groups. Among these, APTES and other amino-alkoxysilanes catalyze the silanization due to their basicity and normally react faster than other nonbasic alkoxysilanes.[69] As a result, uneven coverage may occur;
The grafted organic groups are likely to be very close to each other if a full monolayer coverage is achieved. The distance between groups was estimated to be less than 1 nm.[70] Therefore, steric hindrance may occur and a good assembly of multibiofunctionality (using large biomolecules) may be difficult. This will result in a low loading of biomolecules and the larger the biomolecule, the less effective in binding it becomes.
Nonetheless, cocondensation is a simple way to assemble a chemically stable, multifunctional surface through chemical bonding. It is a one-step method with a relatively short experimental time (usually within 24 h) and the product is easy to isolate. This is still a new concept to researchers working on MNPs because, at the current status, research on silanizing MNPs tends to be associated with APTES type amino-alkoxysilane only. However, such a concept has been demonstrated on a silica surface of nanoporous silica SBA-15.[71] Theoretically, the same chemistry should be applicable to iron oxide MNP surface.
Secondary Reactions of Grafted Groups
A multifunctional surface of MNPs can also be built from a monofunctional surface through secondary reactions, such as formation of amides and imines from primary amines on the surface (Figure 6B). From the previous section, we have learnt that MNPs silanized with a monolayer of organic groups can be prepared using an alkoxysilane. Similar to many organic compounds, the grafted organic groups can undergo secondary reactions and be converted to secondary organic groups (Figure 7D). In other words, this is to convert the monofunctional groups on the MNPs into other groups through chemical reactions. For example, primary amines can react with glutaraldehyde (sometimes termed as glutardialdehyde) to form imines with an aldehyde end groups (Figure 6B). Bear in mind that the yield of organic reactions is often lower than 100% and it is significantly lower for a solid/liquid interface reaction. Consequently, only a partial conversion on the primary groups will occur and the resultant MNPs will posses both the primary and secondary groups on the same surface. This new strategy has recently been demonstrated by Olariu et al. when a bifunctional surface of both amine and carboxylic acid groups were constructed using a reaction between succinic anhydride and amine-functionalized MNPs (prepared with APTES).[72] A conversion of approximately 30% was recorded and this gives a surface of 70% amines and 30% carboxylic acid. Such a contrast in functionalities allows the binding of both RITC (onto the amine groups) and an antibody (onto the carboxylic acid groups) on the MNPs and results in a multimodal nanomaterial. Although the aim of this work is targeted at use in cancer diagnosis, the protocol can be applied to manufacture multifunctional MNPs for other biomedical applications (drug delivery or gene binding).
In other cases, the amount of secondary functionality could cause unexpected effects. For example, Bouffier et al. grafted a DNA intercalator PyAcr (9-chloro-4H-pyrido[4,3,2-kl]acridin-4-one) onto the terminal -NH2 of diethylenetriamine groups,[70] which was already found to bind DNA through electrostatic interaction [Yiu HHP et al., Manuscipt Submitted]. The DNA binding capacity increases (over 40%) when the sample has approximately 20% of the diethylenetriamine groups grafted with the PyAcr group despite a significant drop on zeta potential. However, a fully grafted sample has shown a very low DNA binding capacity and a negative zeta potential (i.e., a negatively charged surface), which is unlikely to attract negatively charged molecules, such as DNA.
Despite not being demonstrated, it can be expected that a third functionality can be built with reaction of the secondary groups, or even the primary groups, in this system. Such possibility shows that this bifunctional system can have a huge potential for assembling multifunctional nanomaterials. Another advantage is that, without the thick polymer shell, the overall size of these multifunctional MNPs can be kept to a minimum, with the coating usually approximately 1–2 nm thick (5–10 carbon atoms plus the -Si-O- base).
Chelating Ligands of Dopamine & Phosphates
There are misunderstandings that thiolated species, which interact strongly with a gold surface, can be used to functionalize unmodified iron oxide nanoparticles. Unlike gold, iron oxides do not have strong affinity to sulfur atoms. Instead, diphenols can chelate onto iron surface strongly, and this can be exploited to functionalize the MNP surface. Only one specific example has been found in the literature exploiting this chemistry for functionalizing MNPs. Dopamine, a diphenol compound with an ethylamine group attached to the benzene ring, was used to cap MNPs.[73] Although the interaction is expected to be weaker than a covalent bond such as Fe-O-Si, this should create a thin monolayer of amine groups, similar to silanization using APTES. The main advantage of using dopamine over APTES is that the reaction temperature is lower as the ligands self-assemble onto the MNP surface. However, without permanent, covalent bonds between ligands and the MNP surface, the ligand shell is expected to be less stable than that of silanized MNPs and the choice of functionalities is very limited.
Phosphate is another type of ligand that has such a property. A recent work published by Benyettou et al.[74] demonstrated the use of cancer-targeting hydroxylmethylene bisphosphonates (HMBPs) to chelate the γ-Fe2O3 MNPs; some of these ligands were bound to rhodamine, creating a bimodal fluorescence-MRI imaging capacity. Although it demonstrated some selectivity towards cancer cells, use of phosphonate binding agents will always suffer from the problematic competition against other phosphate species, particularly phosphate-buffering ingredients in PBS, in the culture medium and even in cell cytoplasm. This will destabilize the functional shell of these phosphonate-coated MNPs and, therefore, they are unlikely to be used in long-term experiments.
Size-exclusion Aided Multifunctional Assembly: A Special Case
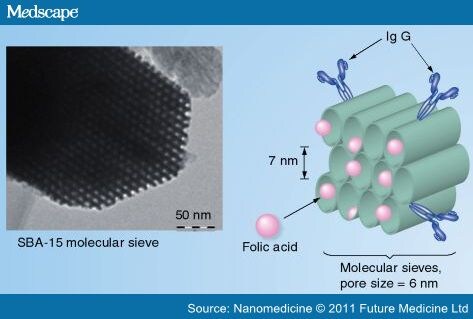
Functional biomolecules can have a wide range of dimensions in size, from small target molecules (e.g., folic acid) of approximately 1 nm, to a large antibody IgG of approximately 10 nm. It is possible to separate them and bind them onto different compartments of a particle using 'size exclusion' if the particle has a well-defined porous structure and a pore size less than 10 nm. This phenomenon is illustrated in Figure 8, and such separation can be carried out with specific 'molecular sieves' of the adequate dimensions. The family of nanoporous materials, M41S, is ideal for this task. Discovered in the early 1990s, M41S are nanoporous silica particles with a highly ordered structure and a narrow pore size distribution (˜0.5 nm) and have been used for drug delivery.[75] Moreover, there are several 3D nanoporous structures (MCM-48, SBA-1) and both the particle size and the pore size can also be tuned with simple physical or chemical alternations during synthesis. For example, SBA-15 nanoporous silicas, depending on the method of synthesis, have a pore size of 6–8 nm (with a pore size distribution of ˜0.5 nm). As such, small molecules can access and interact with the organic groups inside the channel, while large molecules, such as antibodies or large proteins (BSA for example), can only be bound on the outside of the particle.[76] As a result, the whole particle can be made bifunctional with only one type of organic group, small functionalities inside the pores and larger functional molecules bound on the outside.
Figure 8.
Use of molecular sieves for selective binding of biomolecules of different molecular dimension.
The inset is a TEM micrograph for a SBA-15 molecular sieve, with a highly ordered 2D hexagonal porous structure (pore size ˜7 nm).
Furthermore, two members of the M41S family, SBA-16 and KIT-6, have a structure of cages with a 3D network of interconnected channels. The cage sizes are approximately 5–6 nm, which provides an ideal cavity for the growth of MNPs and makes the whole composite particle magnetic. On the other hand, the channels are still vacant for further functionalization. This complex assembly was recently demonstrated using SBA-16, which has a cage size of approximately 6 nm and a pore size of approximately 3 nm.[77] MNPs were synthesized inside the cages with wet impregnation method. The surface (both internal and external) of the resultant FeOx-SBA-16 composite material was grafted with -NH2 groups via silanization.[77] It was found that the groups are capable of binding negatively charged drugs, such as ibuprofen and aspirin. Large proteins, such as IgG and BSA, were found to be bound on the outside of the composite particles, while the -NH2 groups in the channels remained active. Unfortunately, the magnetic core is built on a silica template and the magnetic susceptibility is relatively low due to poor crystallinity.
Toxicity & Safety Issues
One of the major obstacles for advancing functionalized MNPs into commercial diagnostic and therapeutic agents is that the safety issues related to their use in vivo are still unclear. Although there have been clinical trials on MNPs and dextran-coated MNPs have been made available commercially for in vivo use, the safety issues of using functionalized MNPs can be a lot more complicated. This is because the toxicity of functionalized MNPs can be induced from several possible sources: the size and shape of the core materials, the nature of the core materials (magnetite vs maghemite, or other magnetic materials), the nature of the coating materials, the specific biomolecules used, and the methods of functionalization (whether they involve the use of highly toxic reagents). Research papers on functionalized MNPs for biomedicines usually include a basic cytotoxicity test on selected cell lines. Generally, functionalized MNPs were found to be nontoxic at a level of approximately 100 µg/ml for 5000–10,000 cells, but this value does vary greatly. Indeed, this is a rather high tolerance level as it is equivalent to approximately 1 ng of MNPs per cell (or 24 million uncoated/unfunctionalized Fe3O4 MNPs of 25 nm in diameter).[72,78,79] However, these toxicity studies tend to be on a short time frame (1–4 days) and more in-depth biological studies are necessary to provide a clearer picture of the safety of using various functionalized MNPs in vivo.
Nanomedicine. 2011;6(8):1429-1446. © 2011 Future Medicine Ltd.
Cite this: Engineering the Multifunctional Surface on Magnetic Nanoparticles for Targeted Biomedical Applications - Medscape - Oct 01, 2011.




 -SH
-SH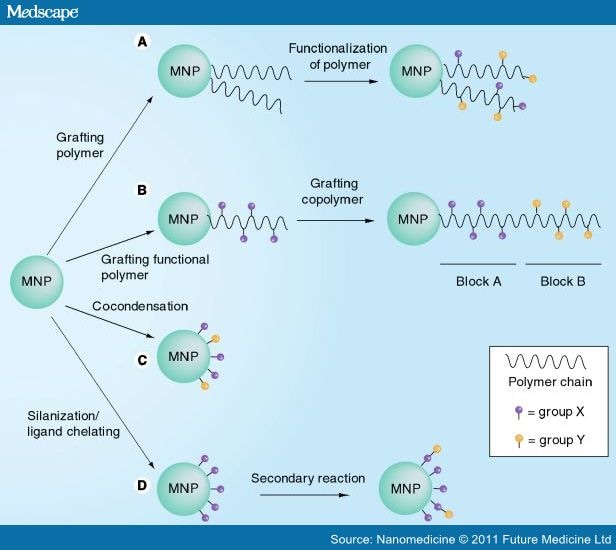








Comments