An apocalypse is looming, warn the public health experts. The spectre of a benighted world where humankind again falls prey to bacterial plagues, wiping out the frail and the young, has been hanging over us for many years now. Infections we have conquered, such as pneumonia and typhoid, will return to kill us. Surgery and chemotherapy for cancer will carry huge risks.
It’s a distant scenario as yet, but it cannot be dismissed as alarmist rhetoric. Antibiotics are no longer the cure-all for bacterial infections that they once were. Antimicrobial resistance is real. Microbes – both bacteria and viruses – are fighting back, developing resistance to the drugs invented to wipe them out. It’s an evolutionary thing. Bugs were here before we were and are evolving to survive us.
Tuberculosis has become a lot more difficult to treat. The TB bacterium has become resistant to more than one of the antibiotics in the cocktail given to patients. In 2016, 490,000 people developed multidrug resistant TB, across every country in the world. To get better, they need newer, more expensive drugs for longer than the standard six months that treatment currently takes. There is every reason to think TB bacteria will develop resistance to the new drugs in time. Control of malaria is also threatened by drug resistance.
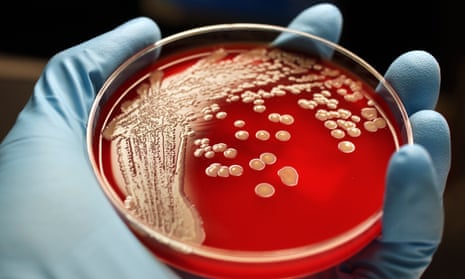
Hospitals in the UK and elsewhere are struggling to treat potentially life-threatening infections caused by Klebsiella pneumoniae, a common bacterium found in the gut. MRSA, the so-called superbug that is a form of Staphylococcus aureus resistant to the antibiotic methicillin, is common. There is growing resistance against drugs used to treat the sexually-transmitted infections gonorrhoea, syphilis and chlamydia. And so it goes on, as the microbes take back the territory they lost to science, bit by bit.
The UK has made a lot of the running, warning the world and urging action. The government convened a major inquiry by the economist Jim O’Neill. The United Nations has held high-level meetings. And now we have a plan launched by the UK health secretary, Matt Hancock, at Davos to incentivise big pharma to get stuck in and find us more antibiotics. It sounds simple. It really isn’t.

Many pharmaceutical companies junked their antimicrobial portfolios some time ago. There was a conviction a few decades back that the war against infectious diseases had been won. Drug manufacturers saw a rosier, more profitable future in chronic diseases. Heart conditions, stroke and type 2 diabetes were and are the big killers today, worsening as obesity levels rise. And unlike infectious diseases, where people take a one-off course of drugs and are hopefully cured, those with chronic diseases could be on the drugs for life. That’s profitable.
There was another issue also causing the pharma bailout. Finding new antibiotic drugs became increasingly difficult. The easy ones had been developed. Companies often like to produce “me too” versions of bestsellers, with slight variations (they would say improvements) to ensure they can get a patent lasting 20 years and recoup their costs. There has been a dearth of new classes of antibiotics for decades.
At Davos, Hancock spoke of incentives to get the companies back in the game. That means the certainty of reward. In 2016, O’Neill recognised this, proposing a $1bn global prize fund for any company that developed a brand-new, needed class of antibiotic.
It makes a lot of sense. A new antibiotic must be put on the shelf and not used until absolutely necessary because all else has failed. That means sales would be very small. Drug companies want blockbusters – usually defined as sales of $1bn. So the O’Neill plan would give them the money up front.
Hancock is not going that far, talking of discussions between NHS England and the National Institute of Health and Care Excellence, which determines cost-effectiveness, to “explore how a new payment model could mean pharmaceutical companies are paid for drugs based on how valuable the medicines are to the NHS – rather than just the sheer quantity of antibiotics sold”.
It’s going to have to be a very generous model and the UK cannot do this alone. Until big pharma sees big numbers and the colour of the money, the difficult challenge of finding the new antibiotics the world badly needs is not one they will readily pick up.