Abstract and Introduction
Abstract
Recent advances in nanotechnology have offered new hope for cancer detection, prevention, and treatment. While the enhanced permeability and retention effect has served as a key rationale for using nanoparticles to treat solid tumors, it does not enable uniform delivery of these particles to all regions of tumors in sufficient quantities. This heterogeneous distribution of therapeutics is a result of physiological barriers presented by the abnormal tumor vasculature and interstitial matrix. These barriers are likely to be responsible for the modest survival benefit offered by many FDA-approved nanotherapeutics and must be overcome for the promise of nanomedicine in patients to be realized. Here, we review these barriers to the delivery of cancer therapeutics and summarize strategies that have been developed to overcome these barriers. Finally, we discuss design considerations for optimizing the delivery of nanoparticles to tumors.
Introduction
Rapid advances in nanotechnology have permitted the incorporation of multiple therapeutic, sensing and targeting agents into nanoparticles (for example, liposomes, viruses and quantum dots), with a size range of 1–1,000 nm. These agents have offered new hope for detection, prevention, and treatment in oncology. Nanomedicine for cancer therapy is advantageous over conventional medicine because it has the potential to enable the preferential delivery of drugs to tumors owing to the enhanced permeability and retention (EPR) effect, and the delivery of more than one therapeutic agent for combination therapy. Other advantages of nanomedicine include specific binding of drugs to targets in cancer cells or the tumor microenvironment, simultaneous visualization of tumors using innovative imaging techniques, enhanced drug-circulation times, controlled drug-release kinetics, and superior dose scheduling for improved patient compliance.[1–6] Furthermore, many widely used conventional chemotherapeutics, such as taxanes, include synthetic solvents (for example, castor oil and polysorbate 80) that directly contribute to adverse effects.[7–9] Finally, many tumor types are inherently resistant to available chemotherapeutics. Nanomedicine has the potential to overcome these problems.[10,11]
Over 20 nanoparticle therapeutics have been approved by the FDA for clinical use.[12,13] Nanoparticle formulations for the treatment of solid tumors (Table 1) include liposomes (such as pegylated liposomal doxorubicin and liposomal daunorubicin), albumin-bound paclitaxel, polymeric particles (such as methoxy-PEG-poly[D,L-lactide] taxol) and many more formulations that are in preclinical and/or clinical trials.[12] Although less toxic than conventional therapies, these agents are still associated with adverse effects, such as stomatitis and palmar–plantar erythrodysesthesia for pegylated liposomal doxorubicin[14] and sensory neuropathy and nausea for albumin-bound paclitaxel.[7] Moreover, these agents are expensive, and the increase in overall survival is modest in many cases (Table 1). Therefore, a better understanding of the barriers that prevent efficacy and uniform delivery of nanoparticles into tumors is needed to develop strategies to improve treatment.
Transport of a therapeutic agent from the systemic circulation to cancer cells is a three-step process. First, nanoparticles flow to different regions of tumors via blood vessels. They must then cross the vessel wall, and finally, penetrate through the interstitial space to reach the target cells. Delivery of diagnostic and therapeutic agents differs dramatically between tumor and normal tissues because of differences in their structure. The abnormal organization and structure of the tumor vasculature leads to tortuous and leaky vessels and heterogeneous blood flow.[15,16] In addition, the lack of functional lymphatic vessels and the vascular hyperpermeability inside tumors results in interstitial hypertension.[17] This uniformly elevated interstitial fluid pressure (IFP) reduces convective transport, while the dense extracellular matrix hinders diffusion.[18] In this Review, we discuss the barriers to nanomedicine delivery and present strategies to overcome them. Finally, we propose design considerations to optimize delivery of nanotherapeutics to solid tumors.
© 2010 Nature Publishing Group
Cite this: Delivering Nanomedicine to Solid Tumors - Medscape - Sep 14, 2010.


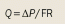
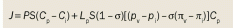
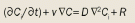




Comments