Medical Therapy
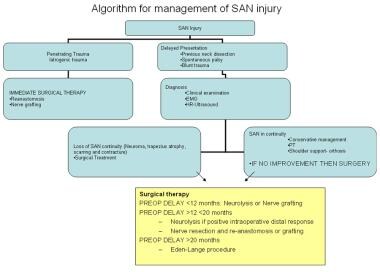
The approach to management of spinal accessory nerve (SAN) injury and trapezius muscle dysfunction is a multidisciplinary approach that involves conservative management, physical therapy, and surgical repair. See below for an algorithm regarding the management of SAN injury.
Indications for nonsurgical management include the following: [15]
-
Serial clinical evaluation that shows improvement in shoulder function
-
Demonstrable clinical/electromyography (EMG) improvement that indicates electrical regeneration
-
Mild, tolerable subjective symptoms; pain; and minimal scapulohumeral dysfunction
Short-term options for nonsurgical therapy include the following:
-
Nonsteroidal anti-inflammatory drugs (NSAIDs)
-
Transcutaneous nerve stimulation
-
Regional nerve-blocking procedures with local anesthetics
These options may have short-term benefits for relieving shoulder pain and improving function. The major drawbacks of short-term treatment options are that the efficacy is highly variable and short lived and nerve-blocking procedures require frequent repeat injections. This makes nerve-blocking procedures unfeasible for long-term management. [47]
Rehabilitation
Rehabilitation in the form of physical and occupational therapy can provide significant functional benefit and improvement of pain. The goal of rehabilitation is to promote wide passive range of motion and gradually active motion to prevent shoulder dysfunction due to adhesive capsulitis or frozen shoulder. [6]
Maintaining proper shoulder alignment is extremely important for effective rehabilitation because it reduces the mechanical tension on the scapula and shoulder girdle. Elevation and medialization of the scapula reduces the level of functional deficit of the trapezius muscle. [48]
The affected limb can be "unloaded" with the following recommendations to the patient: [21]
-
Avoid carrying heavy weights on the affected side.
-
Hook the thumb in a pants pocket or put the hand of the affected side in the pocket, which can provide significant relief.
-
Use an arm sling to reduce pain; however, this can be counterproductive because it can prevent functional activities of the affected arm.
Orthoses (the Akman-Sari orthosis; see the image below) have been used for rehabilitation.
An orthosis provides pain relief and corrects malalignment, promoting appropriate positioning and better functioning of the remaining scapular musculature.
In one study, use of the orthosis has been associated with improvement, in terms of pain and ability to resume daily activities, in shoulder dysfunction after radical neck dissection in which the SAN was resected. In spite of this apparent improvement, the active shoulder abduction improved by only 5-25°; therefore, the improvement must not be mistaken for a return of trapezial function. [48]
Physical therapy (PT)
PT is a crucial component of recovery from SAN injury and shoulder dysfunction. It is essential both for patients who decline or are ineligible for surgical intervention and for those who are planning to undergo surgical repair of the SAN.
The goal of PT is to maintain or regain passive ROM about the shoulder. This serves to limit or prevent stiffness of the shoulder capsule and ligaments that can arise with malalignment of the shoulder and adhesive capsulitis. A PT protocol as suggested by Salerno et al is described as follows: [6]
-
Passive forward elevation of the arm in the plane of the scapula in supine and half-sitting positions
-
Passive forward elevation with the hands locked in supine and half-sitting positions and subsequent stretching movements
-
External rotation with the elbow at the side and flexed at 90°
-
Internal rotation with the hand placed behind the back
The investigators assigned physical therapy to one study group (PT group) and compared that group’s findings with the findings of another group that did not undergo physical therapy (non-PT group). [6] All patients had undergone neck dissection with anatomic preservation of the SAN. A significant improvement in mobility, pain, quality of life, and return to previous occupation was seen in the PT group. Approximately 63% of the PT group, as compared with 10% of the non-PT group, was able to achieve what is known as the zero position: 165° flexion at the shoulder joint in a plane 45° anterior to the coronal plane. Achievement of this position is necessary for common daily activities.
Note that the benefits of PT were not evident 1 month after initiating therapy but were significant after 6 months of aggressive PT. [6] The authors of this study emphasized early and prolonged PT, beginning within 1 month of surgery and lasting, on average, 3 months. Optimally, PT should be instituted within 1 month surgery and continue for at least 3 months; however, recent literature suggests that PT is also effective for late-diagnosed SAN injury. [49]
A randomized, controlled trial by McGarvey et al suggested that scapular strengthening exercises following SAN injury in neck dissection can, at least in the short-term, maximize shoulder abduction. In the study, patients who had undergone neck dissection received either 12 weeks of progressive scapular strengthening exercises or usual care. At 3-month follow-up, carried out on 52 patients (53 shoulders), shoulder abduction in the PT group was greater than in the usual-care patients by a statistically significant amount (+26.6 degrees). [50]
Ultimately, if nonsurgical management of the shoulder syndrome is pursued, aggressive PT is vital to long-term preservation of shoulder function and reduction of pain. Serial clinical examinations and EMG monitoring is useful to monitor SAN function. However, with a dense, stable clinical and electrical neural deficit, early operative intervention yields the best results. [15]
Surgical Therapy
The decision to undergo surgical intervention in the absence of an obvious nerve injury or resection should be made only after sequential evaluations following the initial trauma fail to show spontaneous improvement in trapezius muscle function and confirm stable neurologic deficits.
Indications for surgical management are as follows: [15]
-
Dense paralysis
-
Absence of clinical or electrophysiologic improvement on serial examinations
-
Distressing subjective symptoms, pain or severe scapulohumeral dysfunction
-
Intraoperative identification of a nerve-in-continuity that does not show contractions on direct stimulation (nerve resection and reconstruction should be considered.) [41]
The surgical options are as follows:
-
Neurolysis
-
Primary nerve anastomosis
-
Cable graft (autograft, biosynthetic nerve guide or Neurotube)
-
Eden-Lange muscle transfer
Although serial examinations may differentiate patients who are appropriate for surgical reconstruction of the SAN from those patients who may be managed conservatively, in the case of clear SAN transection, immediate reconstruction at the time of nerve injury is recommended. In patients who present with trapezius muscle palsy with or without a history of neck surgery, the evaluating surgeon must determine the length of time that has elapsed from the initial onset of symptoms.
Previous studies have emphasized that the best outcomes of surgical repair occur if the surgery is performed within 3 months of the injury. [51, 52] Although this is a rational stance, other studies have reported good outcomes of SAN repair in patients with symptom duration of less than 20 months. Maldonado and Spinner reported on the successful use of a lateral pectoral nerve transfer, employing a supraclavicular approach, for SAN repair in two patients who presented 8 and 10 months postinjury. [53] The Eden-Lange muscle transfer procedure is recommended for cases of spontaneous trapezius palsy or cases in which the elapsed symptomatic time is greater than 20 months. [41]
Neurolysis
Neurolysis is an option for improving nerve function if no anatomical break in continuity of the nerve is found intraoperatively and conduction of electrical impulses is present. Intraoperative stimulation of the SAN should demonstrate transmission of an action potential to the neuromuscular junction, as indicated by trapezius muscle contraction. If the SAN is able to conduct nerve impulses, an extrafascicular neurolysis may be performed. [15, 41] While performing a neurolysis of a scarred segment of the nerve (neuroma), the surgeon must exercise extreme caution because of the risk of damage to underlying functional nerve fibers.
The completion of the neurolysis can be gauged by identifying redundant functional nerve fibers also known as the "bands of Fontana." Under normal circumstances, these bands protect nerve function through their redundancy by permitting physiological nerve stretching. Consequently, preserving these bands during a neurolysis is important. In addition, intraoperative nerve conduction studies, both proximal and distal to the scarred segment of nerve, aid in identification of functional nerve fascicles. [54]
In summary, minimal dissection and neurolysis of a neuroma facilitated by the above techniques is preferred to protect underlying functional nerve fascicles, while nonfunctional fascicles can be bypassed with grafting procedures similar to the ones described below. [54]
Nerve repair
If the SAN is in continuity and without electrically demonstrable transmission upon direct nerve stimulation, a resection of the nerve with the associated scar or neuroma is performed. This is followed by a primary end-to-end anastomosis. Nerve grafting is performed if the primary anastomosis produces unacceptable tension.
The basic surgical principles of both primary nerve anastomosis and nerve grafting are one and the same, as elegantly outlined in Dvali and Mackinnon in 2003, as follows: [54]
-
Preoperative quantification of motor and sensory function
-
Use of microsurgical technique, including magnification, instrumentation, and microsutures
-
Emphasis on tension-free repair [55]
-
Use of interpositional grafting when tension-free primary anastomosis is not possible
-
Primary repair (anastomosis) when possible
-
Delay in repair for approximately 3 weeks in cases in which primary repair is not optimal (eg, severe crush injury, stretch, or loss of nerve tissue) (This allows the surgeon to identify the true extent of the injured area, which is not always evident in the period immediately subsequent to injury.)
-
Promoting early protected range of motion to facilitate nerve gliding and to prevent contracture formation.
Surgical loupes (with a minimum 4X magnification) and/or operating microscopes should be used for nerve repair. Usually, 9-0 or 10-0 nylon sutures are recommended for nerve anastomosis. [54]
Primary anastomosis and principle of tension avoidance
The proximal and distal free nerve endings are cut cleanly with a No. 15 surgical blade on a firm, flat background, or with a micro scissor, to prepare the ends for re-approximation. One epineural interrupted suture is loosely placed to bring the nerve ends together. The proximal and distal ends of the nerve fascicles are aligned with trimming, when necessary, so that the ends are not buckling. That is, the fascicle ends should lightly approximate one another in one line, rather than tight approximation leading to misdirected fibers. Subsequent interrupted sutures are placed.
Nerve elasticity causes retraction of the proximal and distal nerve segments after injury. This, in addition to the actual nerve injury, may create tension along the site of anastomosis. This tension must be avoided because it will lead to gaps between approximated fascicular ends. Ischemia and increased scarring are also complications of excess tension. [54] Of note, fixed positioning of the limb to alleviate tension is not recommended because stiffness of the joint may result, as well as nerve scarring and gapping when limb movement is eventually restarted.
Another tension-relieving technique that is now less popular involves extensive freeing of the proximal and distal nerve ends from surrounding soft tissue. Nerve mobilization less than 2 cm has been reported as acceptable and will not increase the risk of nerve devascularization. [56]
The intraoperative matching of corresponding proximal and distal nerve fascicles using anatomical, histochemical, and electrophysiological analysis is not fully described here. The reader is directed to Dvali and Mackinnon (2003) for an in-depth description of these techniques. [54]
Nerve grafting (autografts or biosynthetic nerve guides)
Several options are available for nerve repair when primary anastomosis will produce unacceptable tension. Grafting with nonvascularized or vascularized autologous nerves are both viable options. An example of a vascularized graft for SAN repair includes creation of a local flap in which the nerve graft is composed of the proximal sternocleidomastoid muscle and the greater auricular nerve. In this instance, the muscle and surrounding fascia provide vascularization to the nerve graft. [14]
Alternatives to autologous grafting include synthetic nerve guides or conduits. Also, nerve allografting with temporary recipient immunosuppression has been effectively used. However, autologous nerve grafting remains the criterion standard. [54]
The first description of cable grafting for repair of the SAN was reported more than 40 years ago. [3]
Immediate reconstruction of the nerve with microsurgical techniques and cable grafts can result in significant restoration of shoulder function. [57]
Cable grafts are recommended to bridge gaps greater than 2-3 cm. [41, 58]
The average size of grafts can vary from 2-5 cm. [41]
Longer intervals between injury and repair are associated with larger gaps due to nerve scarring and retraction. Longer grafts are associated with worse outcomes due to increased fiber misdirection and defective pruning. [39]
An autograft consists of a nerve segment taken from another nerve within the same patient to be interposed between the 2 cut ends of the SAN. Common donor grafts include the following:
-
Greater auricular nerve
-
Sural nerve
-
Anterior branch of the medial antebrachial cutaneous nerve
-
Lateral anti-brachial cutaneous nerve
-
Thoraco-dorsal nerve
The advantages of biosynthetic nerve guides or Neurotube include the following:
-
Avoidance of a nerve harvesting procedure with associated donor site morbidity
-
High availability of nerve guides in various desired sizes
-
Transparency of the nerve guides (allows easy nerve-end visualization)
-
Rapid and simple procedure
The principles of primary nerve anastomosis also apply to graft interpositioning. Both proximal and distal nerve ends must be cleanly cut so that the fascicles are visible. The nerve graft is interpositioned in the reverse direction (ie, the proximal graft end attached to the distal free end of the nerve and vice versa). Nerve fascicles progressively branch and diverge distally, so reverse interpositioning promotes "funneling" of the regenerating axons from proximal to distal through the graft. [54]
Mayer et al described an SAN repair procedure using nerve fascicles from the upper trunk that are associated with axillary nerve function. In the study’s five patients, the investigators found improvement in the active ROM in shoulder abduction from a mean 55° preoperatively to 151° postoperatively. Moreover, the average pain level, reported as 6.8 preoperatively on the visual analogue scale, fell to 0.8 following surgery. [61]
Because grafting requires nerve axons to regenerate across 2 sites (proximal and distal), outcomes were previously believed to be inferior to those with primary anastomosis (ie, with only one site of end approximation). However, this hypothesis has been disproved; a tension free graft inter-positioning will lead to superior outcomes when compared with a tension-laden primary anastomosis. In addition, thinner nerve grafts such as cutaneous nerve grafts are more easily revascularized than thicker grafts, thus leading to better outcomes. [54]
Eden-Lange procedure
Indications for this procedure include the following:
-
A time interval of over 20 months after SAN injury
-
Failed surgical reconstruction of the SAN
-
Delayed diagnosis of a spontaneous trapezius palsy [41]
The principles of the procedure are as follows:
-
The scapula is stabilized using the levator scapulae and rhomboid muscles, which consequently reinforce the action of the denervated trapezius.
-
The levator scapulae and rhomboid muscles normally insert medially on the scapula. These insertion points are surgically transferred to more lateral scapular targets. [62] As a result, when these muscles contract, the traction stabilizes the scapula, particularly during abduction and anterior flexion of the shoulder (see the image below).
-
Preoperative Details
As discussed above, rapid diagnosis and management of SAN injury is critical because surgical outcomes are dependent on the time that elapses from the initial onset of symptoms or nerve injury to surgical therapy. Physical therapy is always recommended to maintain passive range of motion (ROM) about the shoulder joint and prevent adhesive capsulitis. Surgery is indicated if serial clinical examinations as well as electromyelography (EMG) do not demonstrate SAN function. Electrodiagnostic testing at 12 weeks after onset of SAN injury will show recovering function if the nerve is simply neurapraxic rather than transected. [58] If SAN injury is discovered at the time of the initial operation, immediate surgical repair is recommended.
Intraoperative Details
Specific recommendations related to each type of surgical repair have been discussed in previous sections (see Surgical therapy). Some additional recommendations include the following:
-
A generous incision and wide surgical exposure facilitate identification of the nerve. [63]
-
Anatomic landmarks are critical in identifying the proximal and distal ends of the SAN.
-
Intraoperative nerve stimulation can be a useful tool to identify the SAN.
-
Dissection of the distal stump of the nerve under the deep cervical fascia is relatively avascular and allows direct visualization of the nerve branches as they enter the trapezius. Efforts should be made to identify at least 1-3 branches.
-
Reinnervation of the upper portion of the trapezius is vital for correction of shoulder droop and is of high priority. This prevents the dragging pain associated with shoulder syndrome. The paralysis of the middle and lower portions of the trapezius is partially compensated for by the levator scapulae and rhomboid muscles. [39]
Postoperative Details
The postoperative rehabilitation can vary depending on individual preferences and institutional protocols. However, the goal of postoperative care is early rehabilitation gradually graded from passive to active physiotherapy, along with shoulder immobilization for 2-4 weeks (or up to 6 weeks with casting following the Eden-Lange procedure), to facilitate appropriate shoulder alignment. [64]
Follow-up
Initial follow-up intervals should be every week for approximately 6 weeks and then every month thereafter. Documentation of symptoms and functional recovery should be made at each visit. [15] Aggressive physical therapy is vital after surgery in order to avoid fibrosis of the shoulder capsule and to maximize functional improvement. Physical therapy should continue for at least 3 months following surgery. At 6 weeks postsurgery, recovery of function is expected to be noticeable. On average, maximal recovery is evident at 6.5 months (range, 5-12 mo). [15, 58]
Complications
Nerve graft complications include neuroma formation or fibrotic ingrowth along the graft, preventing proximal axon sprouting and causing failure of graft. This complication may be found in patients who received radiotherapy postoperatively or in patients in which a long and nonvascular graft was used. Another complication is morbidity of the donor site that is associated with harvesting the autologous nerve graft.
Outcome and Prognosis
Several factors affect spinal accessory nerve (SAN) function after injury such as the extent of injury, type of neck dissection, radiation therapy, interval between injury and repair, length or the vascularity of the graft. [44] Nerve repair with rehabilitation reduces pain, improves shoulder function, and quality of life. [1] Maintaining a high index of suspicion and accepting iatrogenic trauma as a possible cause of persistent shoulder symptoms is important. This approach will lead to early intervention, improved outcomes, and a better patient-physician relationship.
A study by Eickmeyer et al found that in 5-year, disease-free survivors of head and neck cancer, those who underwent SAN-sparing neck dissection had a higher level of functioning than did patients who were treated with SAN-sacrificing neck dissection, with shoulder flexion and abduction being poorest in the latter group. In addition, a correlation was found between reduced scores on quality-of-life measures and decreased shoulder flexion and abduction. Patients in the study who underwent no neck dissection had the highest level of function. [65]
A study by Park et al of patients who sustained iatrogenic SAN injury during lymph node biopsy found that, among 41 patients who underwent end-to-end repair and 82 patients treated with graft repair, 90% and 85%, respectively, achieved at least grade 3 recovery, as measured using the Louisiana State University Health Science Center (LSUHSC) grading system. Among 29 patients who underwent neurolysis—all of whom were treated with this modality when the SAN “was found in continuity with recordable nerve action potential (NAP) across the lesion”—more than 95% achieved LSUHSC grade 3 recovery or above. [66]
A study by Göransson et al found that at an average 10.2 years postoperatively, patients who had undergone either neurolysis, direct nerve repair, or nerve grafting for SAN injury achieved improvement in the mean active range of shoulder movement of, respectively, 44° (43%), 59° (71%), and 30° (22%). Atrophy of the trapezius muscle was absent or modest in, respectively, 75%, 44%, and 50% of patients, while pain was absent or controllable in, respectively, 63%, 56%, and 50% of patients. The study involved a total of 37 patients, with postinjury time to surgery ranging from 2-120 months. [67]
Future and Controversies
Performing a diagnostic biopsy on lymph nodes in the posterior triangle is controversial. Most isolated enlarged lymph nodes in young, healthy patients are reactive in nature. Even in established cases of head and neck cancer, only a small percentage of patients present with isolated metastasis to the posterior triangle. [63] Observation or fine-needle aspiration biopsy for persistent lymphadenopathy is reasonable. However, noting that persistent lymphadenopathy may represent relevant pathology such as lymphoma is important. The authors have found diagnostic lymph node biopsies to be very useful in our experience.
The surgeon performing a neck node biopsy must explain to the patient the potential for SAN paresis or palsy. Loupe magnification and bipolar coagulation have been recommended as the standard of care while performing surgery on neck nodes. [58] However, the importance of good surgical technique, knowledge of surgical landmarks, and relevant anatomic variations of the SAN cannot be overemphasized.
A study by Lanišnik et al indicated that intraoperative nerve monitoring of the SAN during modified radical neck dissection can lead to reduced postoperative shoulder disability, benefiting surgeons at the start of their learning curve and as they become familiar with the anatomical variation of the SAN. In the study, patients undergoing the dissection procedure were monitored on one side of the neck but not on the other, with the trapezius muscle demonstrating better EMG scores at 6 months postoperatively on the monitored sides than on the unmonitored sides. [68, 69, 70]
In the authors’ experience, indications for cervical lymph node biopsy include persistent lymphadenopathy after a trial of antibiotics, persistent lymphadenopathy associated with an inconclusive fine-needle aspirate, or the presence of risk factors for head and neck cancer or lymphoma. [15]
A future management measure may involve brief electrical stimulation (BES), which is used to improve recovery after peripheral nerve injury. Barber et al studied the use of intraoperative BES therapy on suspected spinal nerve injury (based on electromyographic changes) in patients who underwent level IIb and/or level V oncologic neck dissection. They found a decreased rate of clinically significant shoulder weakness in individuals treated intraoperatively with BES. [71]
-
Course of the spinal accessory nerve (SAN) in the posterior cervical triangle. DG = posterior belly of digastric muscle; T = trapezius; LS = levator scapulae; IJV = internal jugular vein; black arrow = SAN.
-
Shoulder orthosis for scapulohumeral alignment.
-
Relationship of internal jugular vein to the spinal accessory nerve (SAN).
-
Spinal accessory nerve (SAN) posterior to the internal jugular vein.
-
Eden-Lange procedure.
-
Surgical landmarks for the identification of the spinal accessory nerve (SAN).
-
Shoulder syndrome.
-
Spinal accessory nerve (SAN) traversing a bifurcated internal jugular vein (IJV). (* = carotid artery, yellow arrow = SAN)
-
Algorithm for management of spinal accessory nerve (SAN) injury.
-
Surgical management for spinal accessory nerve (SAN) injury and preoperative delay in diagnosis.