Abstract
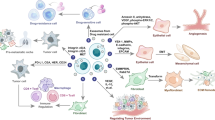
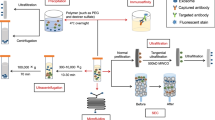
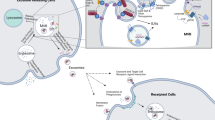
The incidence of cancer is experiencing a steep rise in recent times. A survey report produced by GLOBOCAN 2018 estimates about 18.1 million new cases of cancer across 20 regions of the world. The bewildering number of people afflicted with cancer demands rapid diagnosis and treatment strategy. The current methods used for diagnosis of cancer are expensive, invasive, and time consuming. Hence, a new diagnostic panel has to be laid down to make the process less invasive, cost-effective, and rapid. A venture into identifying potential diagnostic targets introduced exosomes to the scientific community. A plethora of roles being packed into these biological cargoes makes them attractive targets for both therapeutic and diagnostic applications. Exosomes are membrane-bound extracellular vesicles packed with DNA, RNA, and proteins. Their presence in a wide array of body fluids such as breast milk, blood plasma, saliva, urine, serum, and cerebrospinal fluid makes them an excellent source of potential biomarkers. These nano-scale structures are capable of crossing hypoxic regions, systemic circulation and the territories of blood vessel barriers. In line with the above facts, the present review focuses on the therapeutic and diagnostic applications of exosomes in cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–53.
World Health Organization. Global Health Observatory. Geneva: World Health Organization; 2018.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–28.
Kim YS, Ahn JS, Kim S, Kim HJ, Kim SH, Kang JS. The potential theragnostic (diagnostic+therapeutic) application of exosomes in diversebiomedical fields. Korean J Physiol Pharm. 2018;22:113–25.
Suchorska WM, Lach MS. The role of exosomes in tumor progression and metastasis (Review). Oncol Rep. 2016;35:1237–44.
Sharma A. Role of stem cell derived exosomes in tumor biology. Int J Cancer. 2018;142:1086–92.
Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–24.
Valenzuela MM, Ferguson Bennit HR, Gonda A, Diaz Osterman CJ, Hibma A, Khan S, et al. Exosomes secreted from human cancer cell lines contain inhibitors of apoptosis (IAP). Cancer Micro. 2015;8:65–73.
Yang M, Chen J, Su F, Yu B, Su F, Lin L, et al. Microvesicles secreted by macrophages shuttle invasion potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:1–13.
Li L, Li C, Wang S, Wang Z, Jiang J, Wang W, et al. Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver mir-21 to normoxic cells to elicit a prometastatic phenotype. Cancer Res. 2016;76:1770–80.
Steinbichler TB, Dudás J, Skvortsov S, Ganswindt U, Riechelmann H, Skvortsova II. Therapy resistance mediated by exosomes. Mol Cancer. 2019;18:58.
Bebawy M, Combes V, Lee E, Jaiswal R, Gong J, Bonhoure A, et al. Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells. Leukemia. 2009;23:1643–9.
Sousa D, Lima RT, Vasconcelos MH. Intercellular transfer of cancer drug resistance traits by extracellular vesicles. Trends Mol Med. 2015;21:595–608.
Gong J, Luk F, Jaiswal R, George AM, Grau GE, Bebawy M. Microparticle drug sequestration provides a parallel pathway in the acquisition of cancer drug resistance. Eur J Pharm. 2013;721:116–25.
Sun Zhen, Wang Li, Dong Lihua, Wang Xiujie. Emerging role of exosome signalling in maintaining cancer stem cell dynamic equilibrium. J Cell Mol Med. 2018;22:3719–28.
Conigliaro A, Cicchini C. Exosome-mediated signaling in epithelial to mesenchymal transition and tumor progression. J Clin Med. 2018;8:E26.
Quesenberry PJ, Aliotta J, Deregibus MC, et al. Role of extracellular RNA‐carrying vesicles in cell differentiation and reprogramming. Stem Cell Res Ther. 2015;6:153.
McKiernan J, Donovan MJ, O’Neill V, Bentink S, Noerholm M, Belzer S, et al. A novel urine exosome gene expression assay to predict high-grade prostate cancer at initial biopsy. JAMA Oncol. 2016;2:882–9.
Ye SB, Li ZL, Luo DH, Huang BJ, Chen YS, Zhang XS, et al. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget. 2014;5:5439–52.
Fabris L, Calin GA. Circulating free xeno-microRNAs-the new kids on the block. Mol Oncol. 2016;10:503–8.
Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733–41.
Pullan JE, Confeld MI, Osborn JK, Kim J, Sarkar K, Mallik S. Exosomes as drug carriers for cancer therapy. Mol Pharm. 2019;16:1789–98.
Li Y, Zhang Y, Qiu F, Qiu Z. Proteomic identification of exosomal LRG1: a potential urinary biomarker for detecting NSCLC. Electrophoresis. 2011;32:1976–83.
Houali K, Wang X, Shimizu Y, Djennaoui D, Nicholls J, Fiorini S, et al. A new diagnostic marker for secreted Epstein-Barr virus encoded LMP1 and BARF1 oncoproteins in the serum and saliva of patients with nasopharyngeal carcinoma. Clin Cancer Res. 2007;13:4993–5000.
Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–82.
Raimondo F, Morosi L, Corbetta S, Chinello C, Brambilla P, Della MP, et al. Differential protein profiling of renal cell carcinoma urinary exosomes. Mol Biosyst. 2013;9:1220–33.
Welton JL, Khanna S, Giles PJ, Brennan P, Brewis IA, Staffurth J, et al. Proteomics analysis of bladder cancer exosomes. Mol Cell Proteomics. 2010;9:1324–38.
Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO, et al. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009;100:1603–7.
Li J, Sherman-Baust CA, Tsai-Turton M, Bristow RE, Roden RB, Morin PJ. Claudin-containing exosomes in the peripheral circulation of women with ovarian cancer. BMC Cancer. 2009;9:244.
Anami K, Oue N, Noguchi T, Sakamoto N, Sentani K, Hayashi T, et al. TSPAN8, identified by Escherichia coli ampicillin secretion trap, is associated with cell growth and invasion in gastric cancer. Gastric Cancer. 2016;19:370–80.
Rupp AK, Rupp C, Keller S, Brase JC, Ehehalt R, Fogel M, et al. Loss of EpCAM expression in breast cancer derived serum exosomes: role of proteolytic cleavage. Gynecol Oncol. 2011;122:437–46.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that she has no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jayaseelan, V.P. Emerging role of exosomes as promising diagnostic tool for cancer. Cancer Gene Ther 27, 395–398 (2020). https://doi.org/10.1038/s41417-019-0136-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-019-0136-4
This article is cited by
-
Surface protein profiling of prostate-derived extracellular vesicles by mass spectrometry and proximity assays
Communications Biology (2022)
-
Nano pom-poms prepared exosomes enable highly specific cancer biomarker detection
Communications Biology (2022)
-
Cancer nanotechnology: current status and perspectives
Nano Convergence (2021)
-
A Role for Extracellular Vesicles in SARS-CoV-2 Therapeutics and Prevention
Journal of Neuroimmune Pharmacology (2021)
-
The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects
Signal Transduction and Targeted Therapy (2020)