Abstract
To examine the effects of low-intensity resistance exercise training (LIRET) and whole-body vibration training (WBVT) with an external weighted vest on arterial stiffness, wave reflection, brachial flow-mediated dilation (FMD), and physical performance in postmenopausal women. Thirty-three postmenopausal women were stratified by age, body mass index (BMI), and maximal voluntary contraction (MVC) (age, 65 ± 4 years; BMI, 23.3 ± 2.6 kg/m2; MVC, 17.4 ± 2.6 kg) and randomized into LIRET, WBVT, or a nonexercising control group for 12 weeks. Arterial stiffness, augmentation index (AIx), augmented pressure (AP), brachial FMD, gait speed and leg strength were measured at baseline and 12 weeks. WBVT induced improvements in pulse pressure amplification (PPA) (0.04 ± 0.02) compared to control (P = 0.048) and in wave reflection indices [AIx (−4.3 ± 1.4%) and AP (−2.9 ± 1.3 mmHg)] compared to LIRET (P = 0.039 and 0.048, respectively). WBVT (3.8 ± 1.4%) and LIRET (5.0 ± 1.5%) induced similar improvements in FMD compared to control (P = 0.029 and 0.008, respectively). WBVT and LIRET elicited similar increases in leg strength (P = 0.001 and 0.019, respectively), compared to no improvement in the control group. LIRET significantly increased gait speed compared to WBVT (P = 0.043). Although both WBVT and LIRET increased brachial artery FMD (systemic effect), WBVT seemed to be more efficacious in improving wave reflection and cardiac pulsatile load. Interestingly, LIRET elicited a significant improvement in gait speed. Both modalities seem effective in improving systemic endothelial function and muscle strength in postmenopausal women.
Similar content being viewed by others
Introduction
Women are more affected by the adverse impact of increased aortic pulsatile hemodynamic load on the left ventricle due to greater pressure wave reflection than men despite lower arterial stiffness (pulse wave velocity, PWV) [1]. Augmentation pressure (AP), the proposed main wave reflection index [2], is a primary contributor to increased aortic pulse pressure (PP) in older women [3], independent of aortic PWV, which consequently induces a greater left ventricular afterload. Increased afterload may further contribute to the rising prevalence of hypertension and heart failure in postmenopausal women [4, 5]. In addition, impaired endothelial vasodilatory function after menopause [6] is an underlying mechanism for the increase in aortic PP and AP [3]. In nonobese older women, endothelial dysfunction has been associated with lower muscle strength [7], the main characteristic of sarcopenia over low muscle mass and physical performance [8]. Endothelial dysfunction has been associated with sarcopenia, as reduced blood flow impairs anabolic processes necessary to maintain muscle properties [9], in postmenopausal women. Brachial artery endothelial function (i.e., flow-mediated dilation, FMD) is an accepted biomarker of cardiovascular risk prediction and vascular health.
Reduced walking capacity, muscle mass, and muscle strength are associated with increased risk for cardiovascular and all-cause mortality in older adults [10, 11]. Evidence suggests that loss of muscle strength exceeds the loss of muscle mass, a phenomenon more prominent in the legs [12]. Grip strength has a strong association with cardiovascular mortality not explained by reduced muscle mass [13]. Furthermore, normalized grip strength (NGS) to body mass is robustly associated with cardiometabolic disease risk [14] and physical disability [15]. Therefore, interventions that target both skeletal muscle and vascular maladaptations are paramount.
High-intensity resistance exercise training (RET) has shown effectiveness in increasing muscle strength and walking speed [16]. However, concerns about high-intensity RET are potential adverse effects on PWV [17, 18]. These effects appear to be related to intensity and to upper-body, but not lower-body, exercises [17]. Low-intensity RET (LIRET) with slow-contractile speed has been shown to improve muscle strength comparable to high-intensity RET in untrained young adults [19]. Furthermore, LIRET has improved muscle strength, blood pressure (BP), and indices of wave reflection in postmenopausal women [20, 21] and brachial FMD in young, healthy, untrained adults [22]. There are no data regarding the effects of LIRET on FMD in postmenopausal women.
An alternative low-intensity strength exercise modality is whole-body vibration training (WBVT), which utilizes a controlled vibration stimulus while performing leg exercises [23]. Previous findings suggest WBVT ameliorates elevated BP, indices of wave reflection, and arterial stiffness in obese women [24,25,26]. As shear stress levels play a large role in resistance artery remodeling and endothelial nitric oxide (NO) expression [27], WBVT is proposed to improve vascular function (e.g., arterial stiffness, wave reflection, and endothelial function) via increased NO production [24, 28, 29]. Both LIRET and WBVT are effective in increasing muscle strength; however, the ability of either to improve vasodilatory capacity (FMD) and exercise performance in postmenopausal women at risk for sarcopenia is not known.
The purpose of this study was to examine the effects of WBVT and LIRET on wave reflection, arterial stiffness, endothelial function, and physical performance in apparently healthy postmenopausal women. We hypothesize that although WBVT will have a greater effect on aortic hemodynamics, WBVT and LIRET will have equivalent effects on brachial FMD and leg exercise performance, and both interventions will be superior to nonexercise control.
Methods
Participants
Thirty-three nonobese (BMI < 30 kg/m2) women aged 55–75 years volunteered to participate in this study. Participants were postmenopausal for at least 1 year. Exclusion criteria included BMI > 30 kg/m2, history of cardiovascular diseases, renal/pulmonary/metabolic diseases, systolic BP > 160 mmHg, current smokers (or cessation less than 1 year earlier), metal implants, inability to complete the 6-min walk test (6MWT) without stopping, and hormone replacement therapy prescribed within the last 6 months. Participants were also excluded if they were taking beta-blockers or anti-inflammatory drugs and if they were participating in regular resistance or aerobic training. Due to the negative relationship between heart rate and augmentation index (AIx) [30] as well as the disparity of effects on aortic BP and FMD between different generations of beta-blockers [31], participants on beta-blockers were excluded to ensure that our results were accurate. Usage of anti-inflammatory medications, specifically cyclo-oxygenase-2 inhibitors, is associated with elevated cardiovascular risk and foam cell formation, although these data are controversial and have been for a few decades [32]. To maintain homogeneity within our sample, it was essential that participants taking anti-inflammatory medications be excluded due to the potential differences in duration of use. The study protocol was approved by the Institutional Human Subject Committee.
Protocol and experimental procedures
We used a parallel experimental design. Following an initial screening and familiarization session, eligible women were randomly assigned to WBVT, LIRET, or control for 12 weeks. Randomization was stratified and balanced by age and grip strength. Laboratory personnel were blinded to group assignment. Participants reported to the laboratory in the morning following an overnight fast before and after the 12 weeks (±1 h to avoid diurnal variations), at least 48 h after the last training session to avoid any acute effects of the exercise on the vascular measures (i.e., BP). Participants were asked to abstain from alcohol, vitamins, and dietary supplements for 24 h and from vasoactive medication 8 h prior to testing. Vascular measurements were taken in a dark, quiet, temperature-controlled room (23 °C). Prior to vascular assessments, height, weight, waist circumference (midpoint between lowest rib and iliac crest), and grip maximal voluntary contraction (MVC) were measured. Waist circumference was taken as the average of duplicate measurements that had less than a 5 mm difference. MVC was taken three times, with the highest score recorded from each hand. NGS was calculated as MVC/BMI [15].
Arterial stiffness and wave reflection
Following 10 min of supine rest, PWV was recorded by a semiautomatic device (VP-2000; Omron Healthcare, Vernon Hills, IL), which uses BP cuffs around both arms (brachial artery) and ankles (posterior-tibial artery) and tonometers over the right carotid and femoral arteries to obtain brachial-ankle PWV (baPWV), carotid-femoral PWV (cfPWV), and femoral-ankle PWV (faPWV). The feet of the pulse waves are related to the ECG’s R-wave to calculate transit time. The distance between sampling points for cfPWV was measured with a segmometer, whereas for baPWV and faPWV, this value was calculated automatically according to the subject’s height. PWV was calculated as distance/transit time, while heart rate (HR) was obtained from the ECG. PWV measurements were taken in duplicate with less than 5% difference between measures. A third measurement was recorded when the difference was greater than 5%.
Radial applanation tonometry assessed wave reflection through pulse wave analysis. Brachial BP, measured in duplicate with an automated device (Omron HEM-907XL Pro Healthcare, Vernon Hills, IL) following ~20 min of quiet rest, was used to calibrate radial waveforms obtained from a 10-s epoch using a high-fidelity tonometer (SPT-301B; Millar Instruments, Houston, TX). Aortic pressure waveforms were derived using a generalized validated transfer function (SphygmoCor, AtCor Medical, Sydney, Australia). PP was calculated as the difference between systolic (SBP) and diastolic BP (DBP). Pulse pressure amplification (PPA) was depicted as the ratio of brachial to aortic PP [33]. AP was calculated as the difference between the first and second systolic peaks. AIx was calculated as the ratio of AP–PP and was standardized at 75 bpm (AIx@75) given the inverse relationship between HR and AIx [30]. Measurements were taken in duplicate with less than 5% difference and an operator index >80.
Endothelial function
Brachial artery diameter and mean blood velocity (MBV) were measured using Doppler ultrasound (Philips HD11XE; Philips Ultrasound, Bothell, WA, USA). A 12–2 MHz linear-array probe was held in place 1–5 cm proximal to the antecubital fossa at an insonation angle <60°. Following baseline measurements, a cuff on the upper forearm was inflated to at least 50 mmHg above SBP for 5 min. Measurements were recorded continuously from 30 s prior to the release of the occlusion pressure to 3 min after cuff deflation. FMD was calculated as the maximal postocclusion diameter relative to the averaged preocclusion diameter.
Brachial artery diameter was analyzed in late diastole using ECG-gated automated edge-detection and wall-tracking software (Medial Imaging Applications; Coralville, IA, USA). All videos and images were recorded and saved to an offline computer for future analysis. Each sequence of images was visually reviewed to ensure that diameters were continually tracked from the intima-lumen interface at the distal and proximal vessel wall. MBV was measured by a full-width pulsed-wave gate, positioning the cursor mid vessel, and detecting the peak of the waveform. Brachial artery blood flow (ml/min) [34] and vascular conductance [35] were calculated as follows:
Strength and physical performance
During the familiarization session, participants performed three leg exercise 1RM tests and a 6MWT. Briefly, the 1RM was obtained by finding the maximum load the participants could move through the full range of motion in the leg press, flexion, and extension (MedX, Ocala, FL, USA) as previously described [36]. For the 6MWT, participants were instructed to walk as fast as possible around a 45-m course. Verbal feedback was given after the completion of every lap, and every minute that passed was announced. The 1RM and 6MWT were repeated after at least 48 h of rest, and the highest value was reported. Both tests were supervised and recorded by the same researcher before and after the 12 weeks.
Exercise training protocol
Participants in the WBVT group performed dynamic exercises for the legs (full squats, high squats, wide squats, and calf raises) on a vibration platform (pro6 AIRdaptive; Health Performance International, Northbrook, IL, USA). Exercises were performed starting from an upright position to 90° and 120° knee flexions (squat) and maximal heel elevation (toe stand). The training volume was increased progressively by increasing the intensity of vibration (24–40 Hz), the number of sets per exercise (2–3), and the total duration of the training session (20–35 min) and by increasing the external load using a weight vest. The vest (1 lb) was worn during the first 2 weeks without external weight.
The LIRET sessions consisted of leg press, leg extension, leg flexion, and calf raise exercises initially prescribed at 40% of the 1RM, with the maximum goal of 15 repetitions. Exercises in both WBVT and LIRET were performed with slow contractions (2-second concentric and 3-s eccentric contractions) controlled with a metronome. Both groups began with 2 weeks of 20-min sessions, while the rest of the training intervention had sessions of 30–35 min. Weight was progressively increased 5–10% if the participant could complete 15 repetitions with a rating of perceived exertion below 7 (Borg CR-10) across 2 consecutive sessions.
Statistical analyses
An estimation of an appropriate sample size was conducted using previous research investigating the effects of LIRET on FMD in young adults [37]. With an effect size of 0.48, an a priori estimated sample size of 21 would enable us to observe a significant difference (2–3%) between treatments (WBVT and/or LIRET vs. control) with a power of 95%. Normality of distributions was tested using the Shapiro–Wilk test for all variables. One-way analysis of variance (ANOVA) was used to detect possible differences between the groups at baseline. Between- and within-group comparisons were performed with a two-way ANOVA with repeated measures (groups (WBVT vs. LIRET vs. control) × time (0 vs. 12 weeks)) with Bonferroni adjustments. When there was a significant group-by-time interaction, one-way ANOVA across change scores was used to detect between-group differences in the responses. Pearson’s partial correlations were used to analyze the relationship between changes in leg strength and walking speed. All statistical analyses were performed using SPSS, version 21.0 (SPSS, Chicago, IL, USA). Statistical significance was considered at P < 0.05. Data are presented as mean ± SEM.
Results
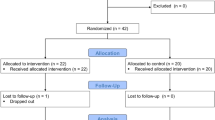
One hundred sixty-seven postmenopausal women were assessed for eligibility, 126 were excluded, and 41 were enrolled in the study. Participants were randomized to the three groups, with two dropping from each training group and four from the control group. Thirty-three participants were analyzed. A Consolidated Standards of Reporting Trials (CONSORT) study design flowchart is shown in Fig. 1. Compliance with supervised WBVT and LIRET training sessions was 94% and 95.7%, respectively. Participant characteristics at baseline are shown in Table 1. There were no significant differences between groups in any baseline characteristic. Following 12 weeks, the WBVT group significantly increased MVC (8.3%) and NGS (7.9%) (P = 0.011), but it was not different compared with the LIRET and control groups. There were no significant changes in BP or anthropometric variables in any of the groups.
Resting and vasodilatory hemodynamic and arterial characteristics
Peripheral BP, central hemodynamics, wave reflection, and arterial stiffness values before and after 12 weeks of the assigned interventions are presented in Table 2. All vascular measures were similar at baseline across all groups. PPA increased (P = 0.026) and AP, AIx (Fig. 2), and AIx@75 significantly decreased in the WBVT over time (P = 0.043, 0.008, and 0.025, respectively). The magnitude of change in PPA (P = 0.048) was significantly greater in the WBVT compared to the control group, and AP (P = 0.048) and AIx (P = 0.039) were significantly greater in WBVT compared to LIRET (Fig. 2). There were no significant differences in brachial BP, aortic BP, cfPWV, faPWV, baPWV, or HR over time in any of the groups.
Changes in a pulse pressure amplification (PPA), b augmented pressure (AP), c augmentation index (AIx), and d brachial artery flow-mediated dilation (FMD) following 12 weeks of the assigned intervention. Values are mean and SEM. *P < 0.05, †P < 0.01 different from baseline (time effect). ‡P < 0.01 different from control (group × time interaction). aP < 0.05 different from LIRET (group × time interaction)
Brachial artery characteristics at rest and responses to FMD before and after 12 weeks are presented in Table 3. There were no between-group differences in brachial artery measurements at baseline. Following 12 weeks, both the WBVT and LIRET groups showed significant increases in FMD compared to the control group (P = 0.029 and 0.008, respectively; Fig. 2d).
Physical performance
Leg strength and walking speed before and after 12 weeks of the assigned interventions are presented in Table 4. Leg press, flexion, and extension 1RM were similar in all groups before training and increased similarly in the WBVT (P = 0.0001, 0.0012, and 0.0001, respectively) and LIRET groups (P = 0.0005, 0.006, and 0.028, respectively) compared to the control group. Walking distance and speed significantly increased in the LIRET group, and the magnitude of increase was significantly greater compared to the WBVT and control groups (P = 0.045 and 0.037); however, these changes were not correlated with the changes in leg strength (r = .131, P = 0.48).
Discussion
The primary purpose of this study was to determine the effectiveness of WBVT and LIRET in improving wave reflection, endothelial function, and physical performance. The key findings and strengths of the present study are as follows: (1) only WBVT improved PPA, AP, AIx, and AIx@75, (2) both WBVT and LIRET increased brachial artery FMD, and (3) LIRET significantly increased walking speed. Taken together, these results suggest that WBVT and LIRET are equally efficacious strength modalities to improve brachial artery vasodilatory capacity, suggesting a systemic effect. Although WBVT reduced indices of wave reflection, it did not affect walking speed.
In the present study, neither training intervention significantly changed resting brachial or aortic BP in predominantly normotensive postmenopausal women. In contrast to these findings, our previous work has shown significant reductions in central and peripheral SBP in young, overweight/obese, normotensive women [38] and in obese postmenopausal women [24, 25, 39] after 6–12 weeks of WBVT. Previous research evaluated prehypertensive or hypertensive subjects. In contrast, our population of interest was nonobese postmenopausal women who were 42.4% normotensive, 21.2% elevated BP, and 18.2% hypertensive. The lack of change in BP under dynamic WBVT can likely be attributed to the relatively normal resting BP.
In a healthy arterial system, wave reflection is an integral component of coronary perfusion, as the reflected wave returns to the aorta during diastole in healthy young adults [40]. The widening of central PP associated with aging in women is largely caused by increased AP, independent of cfPWV [1, 3, 41], resulting in reduced PPA and a consequential increase in end-systolic stress and cardiac hypertrophy [33]. Therefore, while decreases in peripheral and central BP are important, targeting the underlying factors contributing to the increased aortic wave reflection (AP and AIx) and reduced PPA are essential to attenuate the increased risk of heart failure with preserved ejection fraction [1] in older women with hypertension. The WBVT reduced wave reflection indices compared to LIRET. In agreement with the present findings, Wong et al. [24] observed significant decreases in AP, AIx, and AIx@75 in obese postmenopausal women with high BP after 8 weeks of WBVT. Previously, we have shown similar decreases in AP and AIx (−5 mmHg and −10%, respectively) as well as a negative association between changes in leg strength and AP over a 6-week WBVT protocol [25].
Conversely, there were no changes in wave reflection characteristics in the LIRET group of the present study, which is consistent with previous research in obese postmenopausal women [21]. Casey et al. [42] and Taaffe et al. [43] found no reductions in wave reflection indices after moderate-intensity resistance training for 18 and 20 weeks in older adults. With sedentary aging, the reflected wave returns during late systole, resulting in an augmented afterload (AP and AIx) and reduced coronary perfusion and stroke volume [44]. Augmented central pressures induced by increases in AP and AIx are strongly associated with end-organ damage (heart, brain, and kidneys) [45] and cardiovascular events [46]. Although aortic PP was not a significant predictor, elevation of AIx by 10% has resulted in a 31.8% increase in cardiovascular events independent of peripheral BP [47]. Therefore, the reductions in AP and AIx evoked by WBVT, even without an impact on aortic PP, may reduce the prevalence of cardiovascular events in postmenopausal women.
A novel finding of our study is that both WBVT and LIRET increased FMD, which is consistent with findings in other studies using whole-body LIRET or high-intensity RET in young, healthy or prehypertensive adults [22, 37, 48]. This is clinically relevant, as previous literature has reported that for every 1% increase in brachial FMD, cardiovascular risk is reduced by 17% [49]. LIRET, but not WBVT [50] or moderate-intensity RET [42], has improved brachial FMD in healthy young men [22]. This is the first study demonstrating increases in brachial FMD in response to either WBVT or LIRET in this population (of whom 15% of the entire sample were on estrogen therapy). Because these interventions exercised only the leg muscles at low intensity, we hypothesize that the improved brachial artery reactivity was induced by a systemic effect caused primarily by the effect of regular exercise–upregulated NO production/bioavailability [28].
It is important to note that the mean value of FMD was relatively high when compared to that reported for a similar population in the literature. We did not measure any biomarkers, so we are unable to report values that may suggest potential lipidemia or glycemia dysregulation in our sample. Our participants were nonobese, as defined by waist circumference and BMI, and relatively healthy postmenopausal women. Miyazaki et al. [51] displayed a strong, negative correlation between FMD and waist circumference in older adults. Although Moreau et al. [6] found the mean FMD in early- and late-postmenopausal women to be 5–6%, waist circumference in those cohorts was higher than in our cohort, which may explain the uncharacteristically high FMD values in our study. Furthermore, there is a nonsignificant decrease in FMD following the control period. Boyle et al. [52] reported that endothelial function may be significantly reduced by as little as 5 days of physical inactivity in recreationally active men. This may explain the nonsignificant (P = 0.122) reduction in brachial FMD in the control group following 12 weeks of inactivity.
WBVT and LIRET have been shown to significantly increase muscle strength compared to high-intensity RET in untrained sedentary adults [19, 53]. Roelants et al. [54] reported similar increases in leg extension (16.1%) in postmenopausal women following 24 weeks of WBVT and moderate- to high-intensity RET (8–15 RM), suggesting that RET required greater intensity for comparable strength improvements. Conversely, we have previously reported a 24.4% increase in leg strength following 12 weeks of LIRET in obese postmenopausal women [20, 21]. This is the first study comparing these two low-intensity strength modalities. The similarity in strength gains between modalities may exemplify the need to add an external weight to WBVT in postmenopausal women who are normal-weight or slightly overweight to match the relative intensity of LIRET applied in the present study.
Although the participants in the WBVT group had no change, LIRET increased walking speed to a small (~0.06 m/s) but meaningful level [55]. Nunes et al. [56] reported a similar meaningful change (~0.1 m/s) in response to 16 weeks of high-volume (six sets) moderate-intensity (70% of 1RM) RET in postmenopausal women. Vincent et al. [57] reported similar increases in stair climbing speed following 24 weeks of low- (50% 1RM) or high-intensity (80% 1RM) RET in older adults. Given the augmented risk of cardiovascular events and physical disability in older adults, the prospect of having similar benefits from LIRET compared to high-intensity RET is of clinical significance. A potential explanation for the apparent inefficacy of WBVT on walking capacity is a higher baseline value and, consequently, less room for improvement. It is important to note that previous cross-sectional studies have associated lower limb muscular strength with a faster walking speed (Fragala et al. 2016; Inoue et al. [58]). Furthermore, Santos et al. [59] reported that the improvement in walking speed following 8 weeks of whole-body moderate-intensity resistance training was associated with increases in knee extensor strength (r = −0.45) but not associated with favorable changes in body composition. Although our participants significantly increased knee extensor strength, this was not associated with increases in walking speed. This may be due to the different methods used to measure walking speed. Santos et al. [59] utilized a 10-m protocol, whereas ours was longer. Therefore, leg strength has been associated with maximal walking speed; however, this correlation may not be sensitive enough to detect walking speed during longer protocols. Lastly, it is important to note that our population was highly functional (gait speed of 1.85 ± 0.03 m/s) compared to those at high disability risk (<1.22 m/s) in previous studies [55, 56], so these results may not be generalizable to sarcopenic or frail adults.
We attempted to design exercise programs that would be comparable in intensity. For this reason, we are unable to compare absolute loads between the two modalities (WBVT was based on body weight and external weight, while LIRET used conventional weight machines). However, we were able to progressively increase the relative intensity on an individual basis using 15RM and an RPE scale. Notably, our greatest limitation was the lack of detailed physical activity information, whose presence might have shed more light on the matter. In the health history questionnaire, we simply asked for an approximate level of physical activity per week. All the participants were either sedentary or self-reported sparse participation in group exercise classes such as Zumba and Pilates, prior to beginning our interventions. It is important to note that those classes were immediately halted at least 2 weeks prior to their participation in the study (i.e., before baseline measures). However, none of the participants reported regular aerobic or resistance training, the latter of which was our primary concern. Given the relatively high mean FMD values highlighted in the first major comment, our participants may have had higher-than-average physical activity levels at baseline compared to the general population, though we cannot confirm this. Unfortunately, those data were neither recorded nor reported. This study has several strengths, including vascular and muscular benefits following two unconventional strength training modalities for the legs in nonobese postmenopausal women. Our study adds to the current literature that has found that WBVT may be a viable alternative to ameliorate endothelial dysfunction, aortic pressure load, and muscular weakness in nonobese postmenopausal women. To our knowledge, this study is the first to report increases in brachial FMD and muscle fitness (muscle strength and walking speed) in response to LIRET in this population.
In conclusion, WBVT and LIRET are effective modalities to improve brachial artery endothelial function. Although both WBVT and LIRET benefit leg muscle strength, only LIRET improves walking capacity, while WBVT may have a greater benefit on prevention of cardiovascular events via reduction of indices of wave reflection and cardiac pulsatile load in apparently healthy nonobese postmenopausal women. These results suggest that WBVT has a greater benefit on the attenuation of left ventricular afterload, while LIRET may be more appropriate to increase physical performance in postmenopausal women.
References
Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular-arterial interactions. J Am Coll Cardiol. 2013;61:96–103.
Fantin F, Mattocks A, Bulpitt CJ, Banya W, Rajkumar C. Is augmentation index a good measure of vascular stiffness in the elderly? Age Ageing. 2007;36:43–8.
Cecelja M, Jiang B, Spector TD, Chowienczyk P. Progression of central pulse pressure over 1 decade of aging and its reversal by nitroglycerin a twin study. J Am Coll Cardiol. 2012;59:475–83. http://www.ncbi.nlm.nih.gov/pubmed/22281250. Accessed 28 July 2014.
Yoo J-K, Okada Y, Best SA, Parker RS, Hieda M, Levine BD, et al. Left ventricular remodeling and arterial afterload in older women with uncontrolled and controlled hypertension. Menopause. 2018;25:554–62.
Hinderliter AL, Sherwood A, Blumenthal JA, Light KC, Girdler SS, McFetridge J, et al. Changes in hemodynamics and left ventricular structure after menopause. Am J Cardiol. 2002;89:830–3.
Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab. 2012;97:4692–700. https://doi.org/10.1210/jc.2012-2244.
Yoo J, Kim M, Na J, Chun Y, Park Y, Park Y, et al. Relationship between endothelial function and skeletal muscle strength in community dwelling elderly women. J Cachexia Sarcopenia Muscle. 2018;9:1034–41.
Cruz-Jentoft AJ, Bahat G, Bauer JM, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on de fi nition and diagnosis. Age Ageing. 2019;48:16–31.
Timmerman KL, Volpi E. Endothelial function and the regulation of muscle protein anabolism in older adults. Nutr Metab Cardiovasc Dis. 2012;23:S44–S50. https://doi.org/10.1016/j.numecd.2012.03.013.
Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–33. https://doi.org/10.1093/gerona/60.3.324.
Nofuji Y, Shinkai S, Taniguchi Y, Amano H, Nishi M, Murayama H, et al. Associations of walking speed, grip strength, and standing balance with total and cause-specific mortality in a general population of Japanese Elders. J Am Med Dir Assoc. 2016;17:184.e1–184.e7. https://doi.org/10.1016/j.jamda.2015.11.003.
Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol. 2006;61:1059–64. https://doi.org/10.1093/gerona/61.10.1059.
Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–7.
Lawman HG, Troiano RP, Perna FM, Wang CY, Fryar CD, Ogden CL. Associations of relative handgrip strength and cardiovascular disease biomarkers in U.S. adults, 2011-2. Am J Prev Med. 2015;50:677–83. https://doi.org/10.1016/j.amepre.2015.10.022.
Peterson MD, Duchowny K, Meng Q, Wang Y, Chen X, Zhao Y. Low normalized grip strength is a biomarker for cardiometabolic disease and physical disabilities among U.S. and Chinese adults. J Gerontol A Biol Sci Med Sci. 2017;72:1525–31. https://doi.org/10.1093/gerona/glx031.
Turpela M, Hakkinen K, Haff GG, Walker S. Effects of different strength training frequencies on maximum strength, body composition and functional capacity in healthy older individuals. Exp Gerontol. 2017;98:13–21. https://doi.org/10.1016/j.exger.2017.08.013.
Okamoto T, Masuhara M, Ikuta K. Upper but not lower limb resistance training increases arterial stiffness in humans. Eur J Appl Physiol. 2009;107:127–34. https://doi.org/10.1007/s00421-009-1110-x.
Collier S, Kanaley J, Carhart RJ, Frechette V, Tobin M, Hall A. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre- and stage-1 hypertensives. J Hum Hypertens. 2008;22:678–86. https://doi.org/10.1038/jhh.2008.36.
Burd N, West D, Staples A, Atherton P, Baker J, Moore D, et al. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS One. 2010;5:e12033. https://doi.org/10.1371/journal.pone.0012033.
Figueroa A, Vicil F, Sanchez-gonzalez MA, Wong A, Ormsbee MJ, Hooshmand S, et al. Effects of diet and/or low-intensity resistance exercise training on arterial stiffness, adiposity, and lean mass in obese postmenopausal women. Am J Hypertens. 2013;26:416–23. https://doi.org/10.1093/ajh/hps050.
Figueroa A, Arjmandi BH, Wong A, Sanchez-gonzalez MA, Simonavice E, Daggy B. Effects of hypocaloric diet, low-intensity resistance exercise with slow movement, or both on aortic hemodynamics and muscle mass in obese postmenopausal women. Menopause. 2013;20:1–6. https://doi.org/10.1097/gme.0b013e3182831ee4.
Okamoto T, Masuhara M, Ikuta K. Effects of low-intensity resistance training with slow lifting and lowering on vascular function. J Hum Hypertens. 2008;22:509–11. https://doi.org/10.1038/jhh.2008.12.
Figueroa A, Jaime SJ, Alvarez-Alvarado S. Whole-body vibration as a potential countermeasure for dynapenia and arterial stiffness. Integr Med Res. 2016;5:204–11. https://doi.org/10.1016/j.imr.2016.06.004.
Wong A, Alvarez-Alvarado S, Jaime SJ, Kinsey AW, Spicer MT, Madzima TA, et al. Combined whole-body vibration training and L-citrulline supplementation improves pressure wave reflection in obese postmenopausal women. Appl Physiol Nutr Metab. 2016;41:292–7. https://doi.org/10.1139/apnm-2015-0465.
Figueroa A, Kalfon R, Madzima T, Wong A. Effects of whole-body vibration exercise training on aortic wave reflection and muscle strength in postmenopausal women with prehypertension and hypertension. J Hum Hypertens. 2014;28:118–22. https://doi.org/10.1038/jhh.2013.59.
Alvarez-Alvarado S, Jaime SJ, Ormsbee MJ, Campbell JC, Post J, Pacilio J, et al. Benefits of whole-body vibration training on arterial function and muscle strength in young overweight/obese women. Hypertens Res. 2017;40:487–92. https://doi.org/10.1038/hr.2016.178.
Tuttle J, Nachreiner RD, Bhuller AS, Condict KW, Connors BA, Herring BP, et al. Shear level influences resistance artery remodeling: wall dimensions, cell density, and eNOS expression. Am J Physiol Hear Circ Physiol. 2001;2879:1380–9.
Qui F, Liu X, Zhang Y, Wu Y, Xiao D, Shi L. Aerobic exercise enhanced endothelium-dependent vasorelaxation in mesenteric arteries in spontaneously hypertensive rats: the role of melatonin. Hypertens Res. 2018;41:718–29. https://doi.org/10.1249/01.mss.0000536857.57505.13.
Kerschan-Schindl K, Grampp S, Henk C, Resch H, Preisinger E, Fialka-Moser V, et al. Whole-body vibration exercise leads to alterations in muscle blood volume. Clin Physiol. 2001;21:377–82. https://doi.org/10.1046/j.1365-2281.2001.00335.x.
Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525:263–70. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2269933&tool=pmcentrez&rendertype=abstract.
Peller M, Ozieranski K, Balsam P, Grabowski M, Filipiak KJ, Opolski G. Influence of beta-blockers on endothelial function: a meta-analysis of randomized controlled trials. Cardiol J. 2015;22:708–16. https://doi.org/10.5603/CJ.a2015.0042.
Wang D, Wang M, Cheng Y, Fitzgerald GA. Cardiovascular hazard and non-steroidal anti-inflammatory drugs. Curr Opin Pharmacol. 2005;5:204–10. https://doi.org/10.1016/j.coph.2005.02.001.
Safar ME, Blacher J, Pannier B, Guerin AP, Marchais SJ, Guyonvarc P, et al. Central pulse pressure and mortality in end-stage renal disease. Hypertension. 2002;39:735–8.
Walsh LK, Restaino RM, Martinez-Lemus LA, Padilla J, Jaume Padilla C. Prolonged leg bending impairs endothelial function in the popliteal artery. Physiol Rep. 2017;5:1–8. https://doi.org/10.14814/phy2.13478.
Moore DJ, Barlow MA, Gonzales JU, McGowan CL, Pawelczyk JA, Proctor DN. Evidence for the emergence of leg sympathetic vasoconstrictor tone with age in healthy women. Physiol Rep. 2015;3:e12275–e12275. https://doi.org/10.14814/phy2.12275.
do Nascimento M, Januario R, Gerage A, Mayhew J, Pina F, Cyrino E. Familiarization and reliability of one repetition maximum strength testing in older women. J Strength Cond Res. 2013;20:1636–42.
Okamoto T, Masuhara M, Ikuta K. Effect of low-intensity resistance training on arterial function. Eur J Appl Physiol. 2011;111:743–8. https://doi.org/10.1007/s00421-010-1702-5.
Figueroa A, Gil R, Wong A, Hooshmand S, Park SY, Vicil F, et al. Whole-body vibration training reduces arterial stiffness, blood pressure and sympathovagal balance in young overweight/obese women. Hypertens Res. 2012;35:667–72.
Figueroa A, Kalfon R, Madzima TA, Wong A. Whole-body vibration exercise training reduces arterial stiffness in postmenopausal women with prehypertension and hypertension. Menopause. 2014;21:131–6. https://doi.org/10.1097/GME.0b013e318294528c.
Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita Ja, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–45. https://doi.org/10.1161/01.HYP.0000128420.01881.aa.
Cecelja M, Jiang B, McNeill K, Kato B, Ritter J, Spector T, et al. Increased wave reflection rather than central arterial stiffness is the main determinant of raised pulse pressure in women and relates to mismatch in arterial dimensions: a twin study. J Am Coll Cardiol. 2009;54:695–703. https://doi.org/10.1016/j.jacc.2009.04.068.
Casey DP, Pierce GL, Howe KS, Mering MC, Braith RW. Effect of resistance training on arterial wave reflection and brachial artery reactivity in normotensive postmenopausal women. Eur J Appl Physiol. 2007;100:403–8. https://doi.org/10.1007/s00421-007-0447-2.
Taaffe D, Galvao D, Sharman J, Coombes J. Reduced central blood pressure in older adults following progressive resistance training. J Hum Hypertens. 2007;21:96–8. https://doi.org/10.1038/sj.jhh.1002115.
Nichols WW, Denardo SJ, Wilkinson IB, McEniery CM, Cockcroft J, O’Rourke MF. Effects of arterial stiffness, pulse wave velocity, and wave reflections on the central aortic pressure waveform. J Clin Hypertens. 2008;10:295–303. https://doi.org/10.1111/j.1751-7176.2008.04746.x.
Chirinos JA, Zambrano JP, Chakko S, Veerani A, Schob A, Perez G, et al. Relation between ascending aortic pressures and outcomes in patients with angiographically demonstrated coronary artery disease. Am J Cardiol. 2005;96:645–8. https://doi.org/10.1016/j.amjcard.2005.04.036.
Chirinos Ja, Kips JG, Jacobs DR, Brumback L, Duprez Da, Kronmal R, et al. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis). J Am Coll Cardiol. 2012;60:2170–7. https://doi.org/10.1016/j.jacc.2012.07.054.
Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31:1865–71. https://doi.org/10.1093/eurheartj/ehq024.
Beck DT, Casey DP, Martin JS, Emerson BD, Braith RW. Exercise training improves endothelial function in young prehypertensives. Exp Biol Med. 2013;238:433–41. https://doi.org/10.1177/1535370213477600.
Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011;57:363–9. https://doi.org/10.1161/HYPERTENSIONAHA.110.167015.
Weber T, Beijer A, Rosenberger A, Mulder E, Yang P, Schonau E, et al. Vascular adaptations induced by 6 weeks WBV resistance exercise training. Clin Physiol Funct Imaging. 2013;33:92–100. https://doi.org/10.1111/j.1475-097X.2012.01166.x.
Miyazaki S ichiro, Hiasa Y, Takahashi T, Tobetto Y, Chen H, Mahara K, et al. Waist circumference reduction is more strongly correlated with the improvement in endothelial function after acute coronary syndrome than body mass index reduction. J Cardiol. 2010;55:266–73. https://doi.org/10.1016/j.jjcc.2009.11.006.
Boyle LJ, Credeur DP, Jenkins NT, Padilla J, Leidy HJ, Thyfault JP, et al. Impact of reduced daily physical activity on conduit artery flow-mediated dilation and circulating endothelial microparticles. J Appl Physiol. 2013;115:1519–25. https://doi.org/10.1152/japplphysiol.00837.2013.
Delecluse C, Roelants M, Verschueren S. Strength increase after whole-body vibration compared with resistance training. Med Sci Sports Exerc. 2003;35:1033–41. https://doi.org/10.1249/01.MSS.0000069752.96438.B0.
Roelants M, Delecluse C, Verschueren S. Whole-body-vibration training increases knee-extension strength and speed of movement in older women. J Am Geriatr Soc. 2004;52:901–8.
Kwon S, Perera S, Pahor M, Katula JA, King AC, Groessl EJ, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (The LIFE-P study). J Nutr Health Aging. 2009;13:538–44. https://doi.org/10.1007/s12603-009-0104-z.
Nunes P, Oliveira A, Martins F, Souza A, Orsatti F. Effect of resistance training volume on walking speed performance in postmenopausal women: a randomized controlled trial. Exp Gerontol. 2017;97:80–88. https://doi.org/10.1016/j.exger.2017.08.011.
Vincent KR, Braith RW, Feldman RA, Magyari PM, Cutler RB, Persin SA, et al. Resistance exercise and physical performance in adults aged 60 to 83. J Am Geriatr Soc. 2002;50:1100–7.
Inoue W, Ikezoe T, Tsuboyama T, Sato I, Malinoska KB, Kawaguchi T, et al. Are there different factors affecting walking speed and gait cycle variability between men and women in community-dwelling older adults? Aging Clin Exp Res. 2017;29:215–21.
Santos L, Ribeiro AS, Schoenfeld BJ, Nascimento MA, Tomeleri CM, Souza MF, et al. The improvement in walking speed induced by resistance training is associated with increased muscular strength but not skeletal muscle mass in older women. Eur J Sport Sci 2017;17:488–94.
Acknowledgements
We thank Performance Health Systems for providing the vibration machines.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jaime, S.J., Maharaj, A., Alvarez-Alvarado, S. et al. Impact of low-intensity resistance and whole-body vibration training on aortic hemodynamics and vascular function in postmenopausal women. Hypertens Res 42, 1979–1988 (2019). https://doi.org/10.1038/s41440-019-0328-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-019-0328-1
Keywords
This article is cited by
-
Effects of exercise therapy on disability, mobility, and quality of life in the elderly with chronic low back pain: a systematic review and meta-analysis of randomized controlled trials
Journal of Orthopaedic Surgery and Research (2023)
-
Effects of whole-body vibration or resistive-vibration exercise on blood clotting and related biomarkers: a systematic review
npj Microgravity (2023)