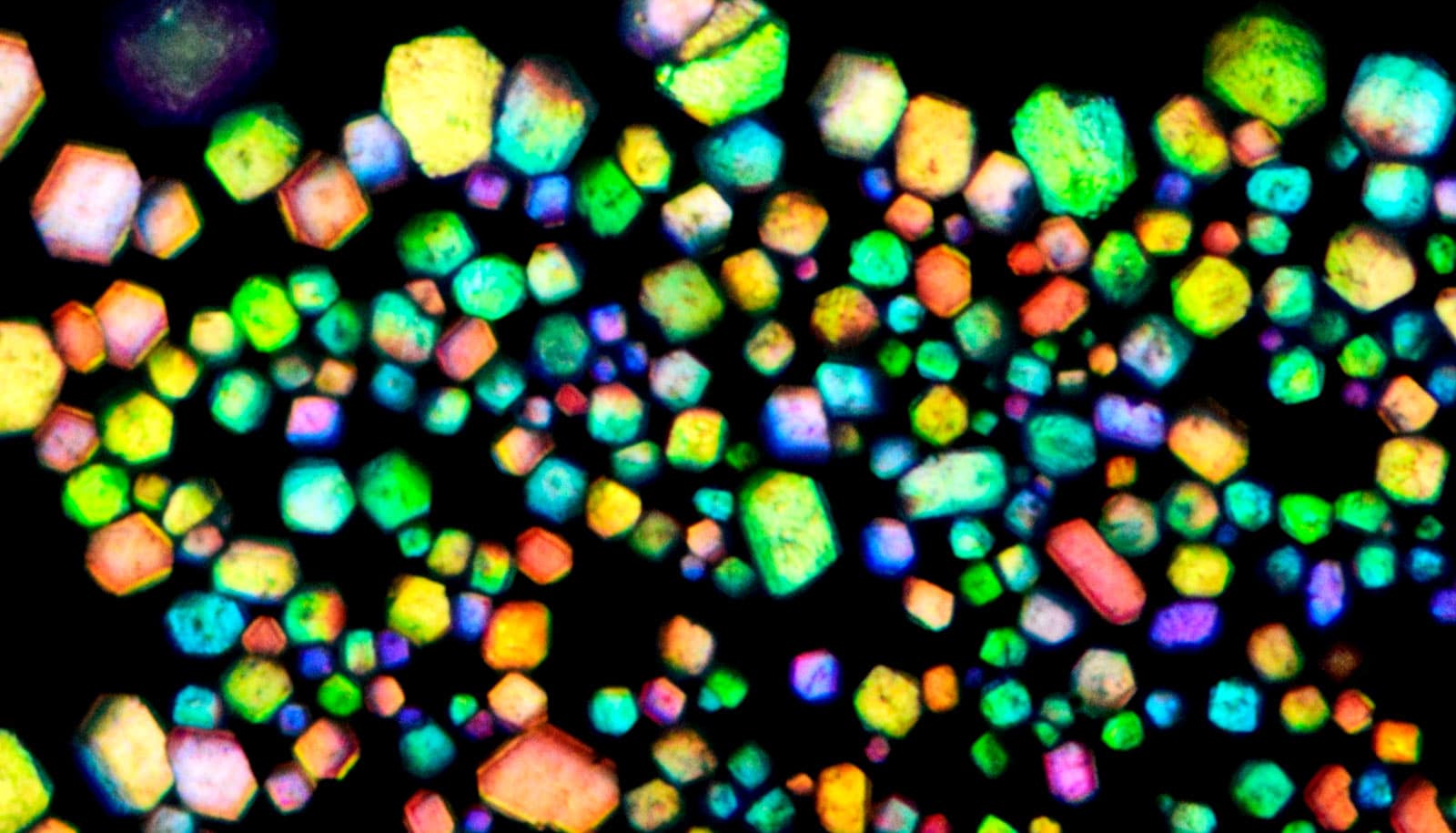
Using just electrostatic charge, common microparticles can spontaneously organize themselves into highly ordered crystalline materials, the equivalent of table salt or opals, according to a new study.
“Our research shines new light on self-assembly processes that could be used to manufacture new functional materials,” says senior author Stefano Sacanna, associate professor of chemistry at New York University.
Self-assembly is a process in which tiny particles recognize each other and bind in a predetermined manner. These particles come together and assemble into something useful spontaneously, after a triggering event, or a change in conditions.
“We’re inspired by nature’s ionic crystals, but we believe we’ll move beyond their structural complexity…”
One approach to programming particles to assemble in a particular manner is to coat them with DNA strands; the genetic code instructs the particles on how and where to bind with one another. However, because this approach requires a considerable amount of DNA, it can be expensive and is limited to making very small samples.
In their study, the researchers took a different approach to self-assembly using a much simpler method. Instead of using DNA, they used electrostatic charge.

The process is similar to what happens when you mix salt into a pot of water, Sacanna explains. When salt is added to water, the tiny crystals dissolve into negatively charged chlorine ions and positively charged sodium ions. When the water evaporates, the positively and negatively charged particles recombine into salt crystals.
“Instead of using atomic ions like those in salt, we used colloidal particles, which are thousands of times bigger. When we mix the colloidal particles together under the right conditions, they behave like atomic ions and self-assemble into crystals,” says Sacanna.
The process allows for making large quantities of materials.
“Using the particles’ natural surface charge, we managed to avoid doing any of the surface chemistry typically required for such elaborate assembly, allowing us to easily create large volumes of crystals,” says first author Theodore Hueckel, a postdoctoral researcher.
In addition to creating salt-like colloidal materials, the researchers used self-assembly to create colloidal materials that mimic gemstones—in particular, opals. Opals are iridescent and colorful, a result of their inner crystalline microstructure and its interaction with light. In the lab, the researchers created their test-tube gemstones with very similar inner microstructures to opals.
“If you take a highly magnified image of an opal, you will see the same tiny spherical building blocks lined up in a regular fashion,” adds Hueckel.
Using electrostatic charge for self-assembly enables researchers to both mimic materials found in nature but also has advantages beyond what naturally occurs. For instance, they can adjust size and shape of the positively and negatively charged particles, which allows for a wide range of different crystalline structures.
“We’re inspired by nature’s ionic crystals, but we believe we’ll move beyond their structural complexity by utilizing all of the design elements uniquely available to colloidal building blocks,” says Hueckel.
The research appears in Nature.
Additional coauthors are from NYU and the University of California, San Diego. Funding for the research came from a National Science Foundation CAREER award and the NSF-funded NYU Materials Research Science and Engineering Center.
Source: NYU