Abstract and Introduction
TORCH infections are unique in their pathogenesis and have potentially devastating clinical manifestations. Congenital toxoplasmosis remains an important cause of blindness, although avoiding exposure to cats and uncooked meat can prevent it. Congenital syphilis has declined in incidence due to mandatory prenatal testing and effective therapy. The incidence of congenital and neonatal varicella and of congenital rubella has been lowered due to vaccination. Perinatally acquired HIV infection continues to increase at a frightening pace in the developing world. The use of antiretroviral therapy in mothers and the newborn, however, has resulted in a decrease in incidence in the United States. While cytomegalovirus remains the most common cause of congenital infection in the United States, the possibility of effective treatment with Ganciclovir (Hoffman-LaRoche, Basel, Switzerland) has emerged from recent studies. In neonatal herpes, selective use of cesarean delivery and antiviral therapy can decrease incidence and improve outcomes.
When a TORCH test or screening is ordered on a newborn, it is suspected that that child has been exposed in utero to one of several organisms that can cause mild or subclinical disease in the mother but devastating damage to the infant. Over the recent past, the organisms involved in this testing have changed in their incidence and outcomes based on new understanding of their pathogenesis, detection, treatment, and prevention. The following article is a review of those pathogens. We will discuss their most common effects and the impact of public health measures, vaccination, molecular biologic techniques, and new therapies.
TORCH, as an acronym, stands for Toxoplasmosis, Other [T. pallidum, Varicella-zoster virus (VZV), Parvovirus B19], Rubellavirus, Cytomegalovirus (CMV), and Herpes Simplex Virus (HSV). Klein and Remington[1] have suggested that this classification is too limiting and that several additional infectious agents should be considered in the Other category, such as enteroviruses, Borrelia burgdorferi (the cause of Lyme Disease), and, of course, human immunodeficiency virus (HIV). This review, however, will only cover the traditional TORCH infections as well as HIV.
The usual way in which the fetus is infected is by transplacental spread after maternal infection in which the organism circulates in the mother's blood. These infections, acquired in utero, can be severe enough to cause fetal loss or can result in intrauterine growth restriction, prematurity, or chronic postnatal infection. In most cases the maternal illness is mild but the impact on the developing fetus is more severe. The degree of severity is dependent on the gestational age of the fetus when infected, the virulence of the organism, the damage to the placenta, and the severity of maternal disease. For example, a primary maternal infection such as herpes simplex is more likely to be vertically transmitted and cause a more severe disease than recurrence of same infection in the mother.[1]
It is difficult to determine the percentage of fetal loss due to infection during early pregnancy. Although fetal loss during the first few weeks of pregnancy has been estimated to be 31% after implantation, this percentage may be misleading. Often women are unaware they are even pregnant when the embryonic death occurs and thus these losses are unaccountable.[2] The earliest recognizable effects of infection are usually seen after six to eight weeks of pregnancy. At this stage it is still difficult to determine whether intrauterine death is due to interference with organogenesis or the systemic effects of infection.
The pathogenetic mechanisms of these infections are unique. Because of their relatively low virulence, the organisms involved seldom lead to fetal death beyond the earliest stages of embryogenesis. Since the fetus is essentially a graft of foreign tissue in the uterus, the placenta constitutes a protective immunologic barrier that shields the fetus from the mother's humoral and cell-mediated immune responses. This makes the fetus especially susceptible to infection during the first trimester and the perinatal period. Early in pregnancy the most complex events in embryogenesis take place, making sensory organs such as the eyes and ears vulnerable. The immature fetus lacks the immunologic mechanisms necessary to completely eliminate an infecting organism. Therefore, a state of immunologic tolerance is often established, which results in persistence of organisms that ordinarily would be eliminated by a normal child or adult.[1,3]
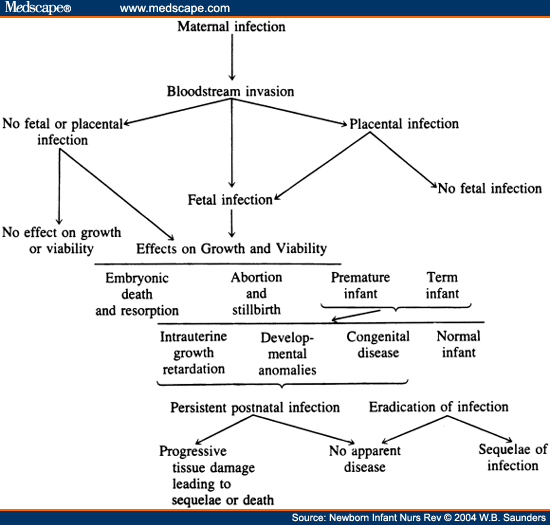
Clinical evidence of infection may be seen at birth, soon afterward, or not until years later. The infected newborn infant may display growth retardation, developmental anomalies, or multiple clinical and laboratory abnormalities. The pathogens vary in whether they cause damage as a congenital agent or during the prenatal period ( Table 1 ). The infection can also lead to the late onset of the disease in what appears to be a "normal" newborn, eg, the development of vision-threatening chorioretinitis in an adolescent with congenital toxoplasmosis. Progressive tissue destruction is seen in rubella, HSV, CMV, toxoplasmosis, and syphilis as the infective agents continue to survive and replicate in the tissues for months or years after initial infection. This is particularly unfortunate when treatment is possible. The sequelae of these diseases can also progress over time, eg, the hearing loss that is secondary to rubella infection can progress or develop even after years of normal hearing. Figure 1, adapted from Klein and Remington,[1] gives a very concise description of the possible outcomes of maternal infection on the developing fetus.
Pathogenesis of hematogenous transplacental infections. (From Klein J, Remington J: Current concepts of infections in the fetus and newborn infant, in Remington J, Klein J (eds): Infectious Disease of the Fetus and Newborn Infant. Philadelphia, PA, Saunders, 2001, p 4).
NAINR. 2004;4(1) © 2004 W.B. Saunders
Cite this: Update on TORCH Infections in the Newborn Infant - Medscape - Mar 01, 2004.






Comments