A scanning probe microscope has been used to exert exquisite control over the types of bonds formed and broken in an organic molecule. ‘This is awesome and very exciting work,’ comments Saw-Wai Hla from Ohio University in the US, who wasn’t involved in the research. ‘From an initial molecule, they produce three totally different molecules, which can be transformed from one to another at will and in a controlled manner with atomic precision.’ He notes that the bent alkyne obtained with a high voltage and the cyclobutadiene ring from the low voltage can be used as intermediates for many directed chemical reactions. ‘This level of selective control is very exciting,’ he says.
‘We form different bonds by applying voltage pulses of different values,’ explains Leo Gross at the IBM Research Laboratory in Zurich, Switzerland. ‘Voltages are applied for a few seconds from the microscope tip located several angstroms above the molecule.’ The team used a bespoke system consisting of a low-temperature atomic force microscope (AFM) and a scanning tunnelling microscope (STM) operating under ultra-high-vacuum conditions. ‘The AFM is needed to take images of the molecular structures with atomic resolution and the STM for electronic characterisation,’ Gross says.
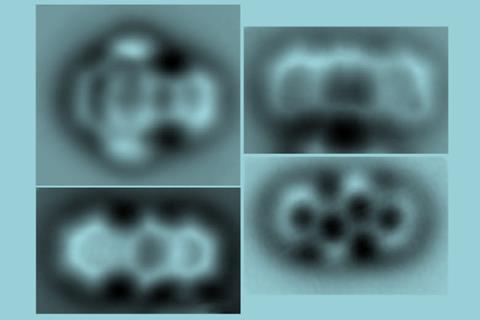
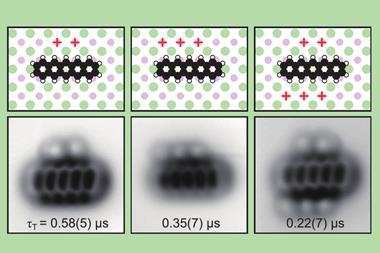
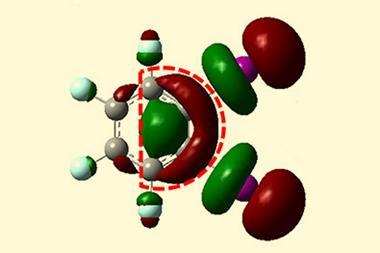
To start, the scientists deposited tetrachloro tetracene, a molecule containing four chlorine atoms attached to a four-ring system, on a thin sodium chloride film on a single crystal copper surface and applied voltage pulses to remove the chlorine atoms. ‘The resulting molecule – a 10-membered ring diradical – turned out to have a very rich on-surface chemistry,’ says Gross’s colleague Diego Peña from the University of Santiago de Compostela in Spain. ‘We found that it’s possible to control which transannular C–C bond is formed depending on the amplitude of the voltage pulse, leading to a four-membered ring fused to an eight-membered ring or to two fused six-membered rings.’
Igor Alabugin at Florida State University, US, who was not involved in the study, points out that the three compounds would have been unstable under ambient conditions. ‘This work reveals chemistry that is unknown and likely impossible under conventional conditions,’ he says. ‘It will challenge organic chemists to reproduce this new chemistry in bulk in either solution or in the solid state.’ He explains that an interplay between kinetic and thermodynamic control provides a way to selectively make one molecule or the other by adjusting the input voltage. And the process is reversible, he adds. ‘Going back to the diradical is uphill in energy and, hence, unfavourable, but the researchers elegantly solve this problem by transiently inverting the relative stabilities via electron injection.’
‘This is the first example of a reversible system with constitutional isomerisation reactions that are selective,’ says Peter Liljeroth from Aalto University in Finland. ‘These experiments offer a very fundamental view on redox reactions that are important for organic synthesis and also for natural processes, at the level of individual molecules.’
Gross notes that they were looking for something else when they made the discovery. ‘We investigated this molecule for a different reason, hoping to see a transformation in which a 14-membered ring was opened.’ But now he thinks that this result might be more interesting. ‘We hope that this approach can be applied to other systems. Now that we understood the mechanism better and have this first example, we will search directedly for other systems that also show such selectivity and maybe even more complexity, possibly leading to molecular machines based on selective bond formation.’
References
F Albrecht et al, Science, 2022, 377, 6603, 298 (DOI: 10.1126/science.abo6471)










No comments yet