Nanofiber Scaffolds for Orthopedic Tissue Repair & Regeneration
Figure 2 illustrates the fiber organizations in various musculoskeletal tissues, including bone, cartilage, tendon, muscle, ligament, tendon-to-bone insertion, meniscus and IVD. These unique anisotropic structures and fibrous architectures cannot be recapitulated by scaffolds fabricated using conventional methods. By contrast, electrospinning can be used to generate nanofiber assemblies for mimicking the special fiber organizations in musculoskeletal tissues. Therefore, electrospun nanofiber scaffolds are capable of mimicking the structural organization of musculoskeletal tissues. As mentioned above, nanofibers could also recapitulate the compositions of musculoskeletal tissues. In the following sections, we mainly illustrate notable examples of the recent advances in nanofiber scaffolds for application in orthopedic tissue repair and regeneration.
Figure 2.
The features of collagen nanofiber organizations in different musculoskeletal tissues.
Collagen nanofibers are uniaxially aligned and wavy in tendon and ligament tissues. Collagen fibers have graded organizations (from uniaxially aligned to random) at tendon-to-bone insertion sites and are circumferentially aligned in meniscus and annulus fibrosus. Cartilage contains fine collagen fibers arranged in layered arrays.
Bone
Bone is a dynamic, highly vascularized tissue and its ECM has two major components: an inorganic phase (constituted by hydroxylapatite) that contributes with 65–70% of bone weight; and an organic phase (composed of glycoproteins, proteoglycans, sialoproteins and bone 'gla' proteins) that comprises the remaining 25–30% of the total bone weight.[76] Collagen fibers overlap adjacent fibers and hydroxyapatite crystals are arranged in layers within each fiber, resembling overlapping bricks. Therefore, the design of nanofiber scaffolds for bone tissue regeneration is often based on the following criteria: highly porous (easy for vascularization); incorporating hydroxyapatite or collagen (mimicking ECM components and mechanical function); and incorporating growth factors (signaling molecules). Electrospun nanofibers are usually deposited on a conductive collector to form a random, porous fiber mat. Furthermore, the porosity can be enhanced using a variety of approaches.[77,78] Incorporation of hydroxylapatite into nanofiber scaffolds can be readily realized by encapsulation or postsurface coating. Collagen itself can be electrospun into nanofibers. In addition, collagen can be blended with other polymers and electrospun to form hybrid fibers. Furthermore, growth factors can be encapsulated inside nanofibers or adsorbed onto the surface of nanofibers. Owing to these intriguing properties, electrospun nanofiber scaffolds have been examined for bone regeneration both in vitro and in vivo.
Nanofiber scaffolds have been shown to support osteogenic differentiation of progenitor cells and stem cells in vitro.[79–82] A number of studies examined the performance of nanofiber scaffolds with different combinations (i.e., incorporating hydroxyapatite, and incorporating collagen and signaling molecule delivery systems) for repairing bone defects in vivo.[83–85] Rainer et al. developed a hydroxyapatite-functionalized PLLA nanofiber scaffold, with the aim to recapitulate the native histoarchitecture and ECM component of sternal bone tissue.[86] Such a scaffold was tested in a rabbit model of median sternotomy. Computed tomography (CT) follow-up confirmed complete healing in the scaffold-treated group 1 week before the control. Histological analysis indicated the formation of new bone trabeculae among implanted nanofibers having a higher degree of maturity compared with the untreated control group. In a separate study, Kolambkar et al. developed a scaffold in combination with an osteogenic signaling molecule delivery system that consisted of a perforated electrospun PCL nanofiber tube and peptide-modified alginate hydrogel injected inside the tube for the sustained release of growth factors (rhBMP-2).[87] This scaffold was tested to repair a critically sized, femoral segmental defect in rats. Figure 3A shows that bilateral 8-mm segmental defects were created in the mid-femoral diaphysis of rats. Prior to defect creation, the femora were stabilized by modular fixation plates consisting of a polysulfone plate and two stainless steel plates. Nanofiber tubes were placed around the adjacent bone ends, such that the tube lumen contained the defect and there was an overlap of 2.5 mm with the native bone ends at each end of the tube. The tube lumen was filled with 125-µl pregelled 2% alginate gel with 5-µg rhBMP-2. Quantitative assessments of bone regeneration were provided by longitudinal 2D radiographs (Figure 3B & C), 3D in vivo µ-CT imaging (Figure 3D & E), torsional testing and histological analysis (Figure 3F). The results indicated that this novel scaffold resulted in consistent bony bridging of the challenging bone defects within 12 weeks. Compared with the intact bone, the biomechanical properties of the repaired bone had statistically equivalent maximum torque and stiffness at week 12. In vivo µCT scanning revealed the presence of perforations in scaffold accelerated early bone formation and defect bridging. In another study, Fu et al. used a composite nanofiber scaffold with incorporation of hydroxyapatite and sustained release of BMP-2 to repair tibia bone defects in mice, demonstrating that the bioactivity of BMP-2 released from the scaffolds was well maintained, which improves new bone formation and the healing of segmental defects.[88] However, these scaffolds could not fully imitate the native structure and compositions of bone ECM. Normal bone function is based on the hierarchical structure at its various length scales. Therefore, the nanofiber scaffold designed for bone tissue regeneration should recapitulate the native structure and composition of bone ECM to a greater extent.
Figure 3.
Electrospun nanofiber scaffolds for bone regeneration.
(A) Implantation of a perforated poly(ε-caprolactone) nanofiber mesh tube filled with a peptide-modified alginate hydrogel containing rhBMP-2 for repairing a segmental defect (8 mm) in the mid-femoral diaphysis of a rat. Modular fixation plates are used to stabilize the femur. Representative radiographs indicating the defect exhibited a robust mineraliztion at (B) week 4, while the defect was bridged with densely packed bone at (C) week 12. (D & E) Microcomputed tomography analysis of bone regeneration at 4 and 12 weeks indicating that the defect was filled with newly formed bone. (F) Ground section stained with Sanderson's rapid bone stain at 12 weeks (four-times) indicating the occurrence of extensive bone deposition throughout the defect and good integration between the newly formed bone and the native bone.
Tendon
Tendons consist of soft and fibrous connective tissue that is composed of densely packed collagen fiber bundles aligned parallel to the longitudinal tendon axis and surrounded by a tendon sheath, also consisting of ECM components.[89] Tendon tissue has a crimped, waveform appearance under microscopic observation. Collagen type I constitutes approximately 60% of the dry mass of the tendon. Owing to the ease of controlling alignment and orders, electrospun nanofiber scaffolds have been investigated in repairing and regenerating tendon tissues. In vitro studies have demonstrated that tendon fibroblasts can adhere and proliferate on electrospun nanofiber scaffolds.[45] Tendon fibroblasts exhibited spindle shape and elongated morphology on aligned nanofiber scaffolds. Nanofiber scaffolds may provide the requisite mechanical properties that may minimize the risk of rerupture associated with the movement of the tendon gap defect following surgical repair. Recently, several studies have been carried out to test the performance of nanofiber scaffolds for tendon repair and regeneration using different animal models.[90,91]
Elements of regenerative medicine consisting of scaffolds, cells and signaling molecules have been combined for tendon injury repair and regeneration. Manning et al. developed PDGF-BB and ASC-loaded nanofiber scaffolds, and tested their performance in repairing tendon defects using a canine model.[90] Figure 4A shows the layered scaffolds that consisted of 11 alternating layers of aligned electrospun PLGA nanofiber mats and a fibrin/heparin-binding delivery system (HBDS). The HBDS allows for the delivery of cells and growth factors in a controlled manner, while the PLGA backbone provides a structure that mimics collagen nanofibrillars and alignment in tendon tissues, and simultaneously enhances the surgical handling properties of the scaffold. Sustained delivery of PDGF-BB was achieved by the HBDS/nanofiber scaffold. The scaffolds were implanted into the sharply transected intrasynovial flexor tendons of adult mongrel dogs. Figure 4B shows the scaffold was grasped by a core structure and secured within the repair site. The dogs were sacrificed at 3 or 9 days postoperatively. The operated tendons were removed by dissection and prepared for histologic analysis at 9 days. Hematoxylin and eosin staining showed no obvious inflammatory response after implantation of the scaffold for 9 days (Figure 4C & D). Only a small number of immune cells infiltrated the scaffold. Histologic studies showed that the HBDS/nanofiber scaffolds were well tolerated and delivery of ASCs was successfully achieved in the in vivo flexor tendon repair setting. In a separate study, James et al. developed a combined treatment of an Achilles tendon gap with tubular electrospun nanofiber scaffolds, ASC seeding and GDF-5 immobilization in rats.[91] However, the prevention of adhesion after repair was not investigated in these studies.
Figure 4.
A layer-by-layer scaffold composed of poly(L-lactide-co-glycolide) nanofibers and a heparin/fibrin-based delivery system containing growth factor PDGF-BB along with adipose-derived mesenchymal stem cells for flexor tendon regeneration in dogs.
(A) The layer-by-layer structure of scaffolds. (B) The scaffold was grasped by a core suture and secured within the repair site. Inset shows that the scaffold was secured within the repair site. (C) Hematoxylin and eosin staining showing no obvious inflammatory response to the implantation of the scaffold 9 days postoperatively. (D) Magnified region in (C) showing only a small number of immune cells infiltrated the scaffold (arrows).
HBDS: Heparin/fibrin-based delivery system; PLGA: Poly(L-lactide-co-glycolide).
Reproduced with permission from [90].
Prevention of tendon adhesion and rerupture after surgical repair to tendon injury remains a clinical challenge. Ni et al. developed a combinatorial approach by combining standard suture and the photobonded electrospun silk nanofiber wrap, which can provide a stronger, adhesion resistant repair, to a tendon injury in adult female New Zealand white rabbits.[92] Biological cues were also incorporated into nanofiber scaffolds for prevention of tendon adhesion and simultaneous promotion of tendon healing. Liu et al. invented a bilayer sheath membrane, consisting of hyaluronic acid-loaded PCL fiber membranes as the inner layer and PCL fiber membranes as the outer layer, for mimicking the tendon sheath, and tested its performance on preventing peritendinous adhesion in a chicken model.[93] It was found that the outer PCL layer is able to reproduce the antiadhesive role of the outer fibrotic layer to reduce peritendinous adhesions, while the inner hydroxyapatite-loaded PCL layer can mimic the biological function of hydroxyapatite secretion to promote tendon healing and gliding. Recently, the same group encapsulated bFGF-loaded dextran nanoparticles in PLLA nanofiber membranes and examined their capability in tendon healing and preventing adhesion in male Sprague–Dawley rats, demonstrating that such membranes can protect the bioactivity of bFGF in a sustained manner for the promotion of tendon healing and simultaneous adhesion prevention.[94]
However, the nanofiber scaffolds designs that are mentioned above failed to recapitulate the wavy structure of collagen fibers in native tendon without loading. Although the wavy-structured nanofiber scaffolds were fabricated, such scaffolds have not been investigated for tendon repair and regeneration in vivo. Here, we highlight the studies on the fabrication of wavy-structured nanofiber scaffolds. Surrao et al. fabricated aligned, wavy poly(l-lactide-co-ε-caprolactone) fibers using a conventional electrospinning setup in conjunction with a rotating wire mandrel.[95] Figure 5A shows a scanning electron micrograph image of nanofiber scaffolds exhibiting aligned and crimpled morphology that were collected with a mandrel at a very high rotating speed. The crimped structure could be preserved even after immersion in phosphate-buffered saline for 4 weeks (Figure 5B). Masson's trichrome staining shows bands of collagen fibers were formed when scaffolds of aligned and random nanofibers were used in vivo at week 6 (Figure 5C & D). Aligned fibers were capable of inducing the alignment of deposited collagen and tendon ECM similar to the native collagen fibrils. It was discovered that the aligned nanofibers, with nano- to micro-meter-sized diameters, underwent crimping after removal from the mandrel. The resulting crimped fibers had similar structural characteristics (amplitude and wavelength) to that of native collagen fibrils. In another study, Liu et al. developed a simple and effective method to fabricate aligned, wavy ester-terminated PLGA and poly(vinyl pyrrolidone) electrospun nanofibers by introducing an external magnetic field to the collector region.[96] Therefore, future studies should be devoted to testing wavy-structured nanofiber scaffolds in animal models for tendon repair and regeneration.
Figure 5.
Fabrication of wavy nanofibers for ligament tissue regeneration.
(A) Scanning electron micrograph image showing a scaffold made of aligned but crimped nanofibers and collected with a mandrel at a very high rotation speed. (B) The crimped structure could be preserved after immersion in phosphate-buffered saline for 4 weeks. (C & D) Masson's trichrome staining showing bands of collagen fibers formed after scaffolds of aligned and random nanofibers were implanted in vivo for 6 weeks. Aligned fibers induced the formation of aligned collagen fibers (arrow) similar to the native collagen fibrils.
Reproduced with permission from [72,95].
Articular Cartilage
Articular cartilage is a type of connective tissue composed of chondrocytes and collagen or yellow elastic fibers, where the fibers and the cells are embedded in a firm gel-like matrix, rich in mucopolysaccharides, exhibiting flexibility and elasticity.[97] The healing of injured cartilage is slow because cartilage has no blood vessels or lymphatics, and the cells' nutrients diffuse through the matrix. Electrospun nanofibers have shown their potential for use in in vivo cartilage regeneration. Toyokawa et al. examined the possible use of two types of electrospun PLGA nanofiber scaffolds, a solid cylindrical type and a cannulated tubular type, for the treatment of a full-thickness articular defect without the addition of exogenous cells in a rabbit model.[98] It was observed that nanofiber scaffolds were absorbed, articular cartilage and subchondral bone were regenerated, and the regenerated cartilage remained present after surgery for 24 weeks. The tubular scaffolds performed better in cartilage tissue regeneration and had higher histology scores than solid ones and the control group, thus, confirming the potential of electrospun nanofiber scaffolds to repair the osteochondral defect without cultured cells and growth factors.
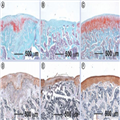
However, nanofiber scaffolds fabricated from the traditional electrospinning procedure hindered cell infiltration due to low porosity. Therefore, there is an imperative need to develop a highly porous nanofiber scaffold that can efficiently entrap cells throughout the entire scaffold and provide the necessary cues to stimulate new tissue development. Cobun et al. developed a low-density, electrospun nanofiber scaffold composed of PVA-methacrylate and a composite of PVA-methacrylate/chondroitin sulfate-methacrylate by making use of an ethanol bath as a collector, and subsequent UV light crosslinking of the methacrylate groups.[74] The chondroitin sulfate that was added to the scaffolds, a compound commonly found in many joint supplements, can serve as a growth trigger. After 42 days of culturing goat MSCs under chondrogenic induction conditions, the scaffolds appeared as discrete cartilage-like constructs, with a shiny appearance similar to hyaline cartilage. Cells proliferated well in the scaffolds and produced collagen with a high ratio of type II:type I collagen. Then, cell-free scaffolds were implanted into damaged cartilage in the knees of rats. Nanofiber scaffolds had significantly more proteoglycan deposition than empty defects but less than native cartilage (Figure 6A–C). Results of immunostaining for type II collagen showed that the scaffolds augmented endogenous articular cartilage regeneration in an osteochondral defect model (Figure 6D–F). The findings in the repaired defects include columnar cell orientation radiating from cell-rich cavities with subchondral bone containing numerous pockets of cellular cavities. Additionally, the early establishment of the multilayer architecture of articular cartilage can be observed, including a gradient of proteoglycan deposition highest near the subchondral bone, and cells oriented perpendicular and parallel to the cartilage surface in the deep and superficial zone, respectively.
Figure 6.
Electrospun poly(vinyl alcohol)-methacrylate/chondroitin sulfate nanofiber scaffolds for cartilage repair.
After implantation for 6 weeks in a rat osteochondral defect model, (A–C) safranin-O staining indicated that (A) the fiber implants promoted significant proteoglycan deposition compared with (B) the negative control (without treatment), while (C) native cartilage had the largest amount of proteoglycan deposition. (D–F) Immunohistochemical staining indicated that even (D) chondroitin sulfate fibers induced higher type II collagen production compared with (E) empty defects, but (F) native articular cartilage still contained significantly more type II collagen.
Reproduced with permission from [74].
Electrospun nanofiber scaffolds in combination with stem cells or chondrocytes were also applied to regenerate cartilage. Shafiee et al. implanted MSCs seeded with electrospun PVA/PCL nanofiber scaffolds into the full-thickness articular cartilage defects of rabbits.[99] After 12 weeks, defects were repaired with chondrocyte-like cells displaying rounded morphology with lacunae. In addition, collagen type II expression increased in the neocartilage. The seeding of stem cells improved healing of defects, compared with the untreated control and those that received cell-free scaffolds. In a different study, Xu et al. developed a five-layer tissue construct of 1-mm thickness with alternate layers of rabbit elastic chondrocytes suspended in a fibrin–collagen hydrogel and PCL nanofiber membrane, using a combination of electrospinning and ink-jet printing.[100] The fabricated constructs formed cartilage-like tissues in vivo as demonstrated by the position of type II collagen and glycosaminoglycans.
However, previous studies have been limited to the examination of nanofiber scaffolds made of random nanofibers on articular cartilage regeneration. The anatomic structure of human cartilage is divided into four different zones, which are termed the superficial, middle (or transitional), deep (or radial) and calcified zones, each with varying matrix composition, morphology, and cellular, mechanical and metabolic properties.[101] The organization of the collagen fibrils in the cartilage presents five features: individual fibrils within the trabeculae join to form small fiber bundles that become grouped into larger bundles at the calcified/uncalcified interface; fibrils in the deep and middle zones, which exhibit the surface periodicity characteristic of collagen, are generally oriented toward the articular surface in large bundles approximately 55 µm across; in the superficial zone, fibrils run parallel to the surface; the surface fibrils have random orientation; and the collagen fibrils of the lacunar walls appear to be thinner and more closely packed than those between the lacunae.[102] Therefore, more appropriate nanofiber scaffolds need to be designed with multiple layers to recapitulate different fiber orientations and compositions in the different zones of native cartilage.
Meniscus
The fibrocartilaginous menisci are load-bearing tissues composed of circumferentially aligned collagen fibers, which are critical to the normal functioning of the knee.[103] Failures in the functioning of the meniscus may occur as a result of traumatic injury or degenerative processes. Repaired tears in the vascular periphery heal well, while those in the avascular inner region fail to do so, and, thus, damaged elements are commonly resected via partial meniscectomy. Removal of tissue results in higher cartilage contact stresses, which may predispose patients to osteoarthritic progression.[104] Replacing damaged regions of the meniscus with an engineered tissue construct may restore function and protect against further deleterious changes in the joint.
Tissue engineering strategies based on electrospun nanofibers present a promising alternative that could allow for regeneration of meniscus tissues. However, no literature has been reported on the use of electrospun nanofibers for menisci tissue regeneration in vivo. Here, we reviewed recent advances of in vitro studies on meniscus regeneration using electrospun nanofiber scaffolds. Mauck's group carried out a series of studies on this topic.[105–108] Their initial study confirmed that MSC seeded, aligned PCL nanofiber scaffolds had great potential for meniscus tissue regeneration.[105] With increasing incubation time, a significantly larger increase in mechanical properties was observed for aligned nanofiber scaffolds compared with random counterparts seeded with meniscal fibrochondrocytes or MSCs. Their subsequent study found that the application of dynamic culture, by incubating constructs on an orbital shaker, dramatically improved the infiltration of MSCs into aligned nanofiber scaffolds.[106] Their further study evaluated the effects of changing fiber angle and sample aspect ratio on the shear properties of aligned PCL scaffolds, and they determined how ECM deposited by resident MSCs modulated the measured shear response.[107] Encouraged by these results, the same group successfully developed a novel electrospinning method to produce scaffolds composed of circumferentially aligned nanofibers using a rotated plate as a collector that could mimic the circumferentially aligned collagen fibers in menisci ECM.[108] Figure 7A & B illustrates the meniscus, showing the generalized anatomic macrostructure and a wedge-like cross section displaying a simplified collagen fiber organization, with the majority of fiber bundles in the circumferential direction, with occasional radial 'tie' fibers. Figure 7C shows scanning electron micrograph images of different locations of circumferentially aligned nanofiber scaffolds. Fibers were locally oriented in these scaffolds, but their orientation varied considerably according to their position. Figure 7D–F, shows fluorescent microscopy images of juvenile bovine MSCs seeded on circumferentially aligned nanofiber scaffolds, with staining of actin in green and nuclei in blue. Juvenile bovine MSCs that were seeded on these scaffolds resulted in a similar orientation to the direction of fiber alignment. Mechanical analysis of these scaffolds revealed significant interactions between scaffold length and region, with the tensile modulus near the edge of the scaffolds decreasing with increasing scaffold length. These scaffolds, with spatially varying local orientations and mechanics, will need to be further tested in animal models.
Figure 7.
Fabrication of circumferentially aligned poly(ε-caprolactone) nanofiber scaffolds for knee meniscus tissue engineering.
(A) Anatomic macrostructure of meniscus. (B) A wedge-like cross-section displaying a simplified collagen fiber organization, with the majority of fiber bundles in the circumferential direction with occasional radial 'tie' fibers. (C) Scanning electron micrograph images showing different locations of samples for circumferentially aligned scaffolds (scale bar: 5 µm). (D–F) Fluorescent microscopy images of actin (green) and nuclei (blue) in juvenile bovine mesenchymal stem cells seeded on the different portions of circumferentially aligned scaffolds.
Ant: Anterior; Lat: Lateral; Med: Medial; Post: Posterior.
Reproduced with permission from [108].
This scaffold designed by Mauck's group can partially mimic the structure of the meniscus by recapitulating its circumferentially aligned collagen fibrous architecture. However, the meniscus is a complex structural tissue, a glossy white, complex wedge-shaped fibrocartilaginous tissue, comprised of cells, specialized ECM molecules, and region-specific innervation and vascularization. The ECM is generated and maintained by meniscal fibrochondrocytes, a heterogeneous cell population sparsely distributed throughout the tissue.[109] Thus, future design of nanofiber scaffolds should be devoted to fully imitating the wedge-shaped, circumferentially aligned collagen fibrous architecture.
Intervertebral Disc
The IVD consists of three tissue components; a gel-like nucleus pulposus (NP) surrounded by the annulus fibrosus (AF), which are sandwiched between cartilage end plates and vertebral bodies, functioning as a ligament to hold the vertebrae of the spine together; a shock absorber; and a 'pivot point' that allows the spine to bend, rotate and twist.[110] The AF is a collagen-rich fibrous structure of approximately 15–25 concentric sheets of collagen (lamellae) that confines the pressurized NP. The lamellar structure of AF, which is composed of collagen type I and II fibers, helps to maintain the tensile properties of the disc while providing structural support for proteoglycan synthesis. The AF has a multilayered, oriented lamellar structure with concentric layers creating a regular pattern of collagen type I fibers. The collagen fibrils are oriented concentrically, with each subsequent layer oriented 60° toward the spinal column. As the outer AF moves inward and approaches the NP, the orientation of the concentric lamellae gradually changes from angles of 62 to 45°. A variety of solid tissue engineering constructs have been investigated for IVD tissue regeneration. However, one major hurdle in the development of scaffolds for IVD tissue engineering is the inability to mimic the lamellar organization of the AF.[111]
In order to address this problem, Nerurkar et al. produced anisotropic nanofiber laminates seeded with MSCs using a layer-by-layer stacking strategy, which could mimic the fibrous architecture of AF tissue to some degree.[112] Figure 8A shows the fabrication of bilamellar-aligned PCL nanofiber scaffolds. In order to replicate the hierarchical structure of the AF, bilamellar tissue constructs were formed first as single lamella tissues from aligned nanofiber scaffolds seeded with MSCs, and then formed into bilayers after 2 weeks of in vitro culture. Electrospun fiber mats, approximately 250 µm thick, can match the natural lamellar thickness of the AF. Rectangular scaffolds (5 × 30 mm) were excised from the nanofiber mat with their long axis rotated 30° from the prevailing fiber direction. Bilayers were oriented with either parallel (+30°/+30°) or opposing (+30°/-30°) fiber alignment relative to the long axis of the scaffold. Sections were collected obliquely across lamellae, stained with picrosirius red, and viewed under polarized light microscopy to visualize collagen organization. When viewed under crossed polarizers, birefringent intensity indicates the direction of alignment. After 10 weeks of in vitro culture, parallel bilayers contained coaligned intralamellar collagen within each lamella (Figure 8B). Opposing bilayers contained intralamellar collagen aligned along two opposing directions (Figure 8C), successfully replicating the gross fiber orientation of native bovine AF (Figure 8D). After an additional 2 weeks of culture, the external supports were removed and laminates remained intact. These scaffolds directed the deposition of organized, collagen-rich ECM that mimicked the angle-ply, multilamellar architecture, and achieved mechanical parity with the native tissue. Their further study identified a novel role for interlamellar shearing in reinforcing the tensile response of biological laminates. In addition, the same group also developed a construction algorithm in which electrospun nanofiber scaffolds are coupled with a biocompatible hydrogel to engineer a MSC-based disc replacement. Similarly, Nesti et al. seeded human MSCs into a novel biomaterial amalgam to develop a biphasic construct that consisted of an electrospun nanofiber scaffold enveloping a hyaluronic acid hydrogel center, which could architecturally resemble a native IVD.[113] Such an engineered IVD was composed of both AF- and NP-like components, demonstrating the resemblance of overall gross, histological, biochemical and biosynthetic properties of a native IVD. Other than mimicking the architecture of IVD, growth factors were also incorporated to nanofiber scaffolds for AF repair and regeneration. Vadala et al. fabricated a bioactive nanofiber scaffold that can release TGF-β1 for AF tissue engineering.[114] The sustained release of TGF-β1 from the nanofiber scaffold could significantly enhance GAG and collagen synthesis in the seeded AF cells.
Figure 8.
Fabrication of bilamellar-aligned poly(ε-caprolactone) nanofibrous scaffold for annulus fibrosus tissue engineering.
Scaffolds were excised 30° from the prevailing fiber direction of electrospun nanofibrous mats to replicate the oblique collagen orientation within a single lamella of the annulus fibrosus. (A) Bilayers were oriented with either parallel (+30°/+30°) or opposing (+30°/-30°) fiber alignment relative to the long axis of the scaffold. Sections were collected obliquely across lamellae, stained with picrosirius red and viewed under polarized light microscopy to visualize collagen organization. When viewed under crossed polarizers, birefringent intensity indicates the direction of alignment. (B) After 10 weeks of in vitro culture, parallel bilayers contained coaligned intralamellar collagen within each lamella. (C) Opposing bilayers contained intralamellar collagen aligned along two opposing directions, successfully replicating the gross fiber orientation of (D) native bovine annulus fibrosus.
Reproduced with permission from [112].
Although some progress was made towards the development of nanofiber scaffolds for AF regeneration, the studies were limited to in vitro investigations. AF has several concentric lamellae of collagen fibers running at angles of approximately 60–80° to the vertical axis.[115] This multiscale structural hierarchy needs to be captured by nanofiber scaffolds in the future. IVD is comprised of AF, NP and cartilage end plates. Perhaps a better design for scaffolds could consist of a combinatorial approach that can integrate hydrogel systems with electrospun nanofibers to fully recapitulate the native architecture of IVD.[116] More efforts should be devoted to testing developed nanofiber scaffolds for IVD regeneration in vivo.
Tendon-to-Bone Insertion Site
As the rotator cuff tendon inserts into the proximal humerus, there is a gradual transition between the four zones at the direct tendon-to-bone insertion: tendon, nonmineralized fibrocartilage, mineralized fibrocartilage and bone. None of the current repair strategies replicate this normal transitional zone, which leads to stress concentrations that weaken the healed tendon-to-bone insertion site, majorly contributing to the high failure rates observed.[117] The stress concentrations are mainly attributed to the mechanical mismatch between the tendon, a soft tissue with a modulus of 200 MPa, and bone, with a modulus of 20 GPa, one of the biggest mechanical mismatches in nature.[118] There are two features at the tendon-to-bone insertion site: gradual organization in collagen fiber orientation and a linear gradient in mineral content from the tendon to the bone. Therefore, a scaffold presenting these two characteristics would be ideal for reducing the concentrated local stress and repairing the injury at the tendon-to-bone insertion site. Accordingly, considerable efforts should be made to develop nanofiber scaffolds that could mimic both the structure and/or composition of the the tendon-to-bone insertion site.[119]
Here, we highlight the nanofiber scaffolds that have been recently designed for the surgical treatment of rotator cuff injury in various animal models. Figure 9A shows that a nanofiber scaffold comprising random poly(glycolic acid) nanofibers was applied to bridge the gap between the infraspinatus tendon humerus bone in a rabbit.[120] Figure 9B shows that another type of scaffold made of aligned PCL and PCL/poly(ethylene oxide) nanofiber were used to augment supraspinatus in rats.[121] After supraspinatus tendon exposure and detachment, scaffolds were implanted using a simple overlay by suturing along the anterior and posterior borders of the scaffold and supraspinatus tendon. The supraspinatus tendon was then repaired back to greater tuberosity. In order to mimic the composition at the bone site, Moffat et al. developed a biphasic nanofiber scaffold composed of two layers of fiber membranes, and then implanted them into the rat shoulder (Figure 9C).[122] In this rat shoulder injury and repair model, the supraspinatus tendon was sharply detached at the insertion site, fibrocartilage at the insertion site was removed and the bony footprint was abraded to mimic the clinical scenario. The scaffold was inserted between the cancellous bone and distal end of the detached tendon. The tendon was sutured to bone. None of these scaffolds could recapitulate either the structural organization of collagen fibers or compositions in the millimeter-sized transitional zone at the tendon-to-bone insertion site, which could partly attribute to the poor functional recovery, such as compromised mechanical performance. In order to mimic the composition at the insertion site, Li et al. developed a continuously graded, bonelike calcium phosphate-coated electrospun PLGA nanofiber scaffold.[123] The mineral content gradient resulted in functional consequences, such as a gradient in the stiffness throughout the scaffold. Recently, Xie et al. successfully fabricated an electrospun PCL nanofiber scaffold with gradations in fiber organization.[45] Additionally, Xie et al. controlled biomineralization of PCL nanofibers using polydopamine as a mediator. Based on these studies, Xie et al. have designed a nanofiber scaffold with dual gradations in both fiber organization and mineral content, and demonstrated the feasibility of performing implantation of such a scaffold to the rotator cuff injury site using a modified rat shoulder injury and repair model (Figure 9D).[124] Specifically, the supraspinatus was detached sharply at its insertion on the greater tuberosity of the humerus. A 0.5-mm drill hole was then made transversely at the base, creating a channel for the scaffold with an exit hole on the proximal lateral humeral metadiaphyseal region. Following suture of the scaffold to the undersurface of the supraspinatus, the tendon–scaffold construct was then secured to the humeral head defect using the 5–0 prolene suture secured at the lateral cortex of the humerus. The fiber scaffold was expected to serve as a graft to provide mechanical stability, and a template to regulate cell activity and improve the healing process. Future studies will be directed toward histological analysis of tissue regeneration and functional recovery tests. In addition, combining stem cell therapy with such scaffolds could be beneficial to the healing of rotator cuff injury, as stem cells could differentiate into multiple cell types at different scaffold portions (i.e., tendon fibroblasts at tendon site, osteoblast at bone site and fibrochondrocytes in between).
Figure 9.
State-of-the-art nanofiber scaffolds for repairing rotator cuff injury in vivo.
(A) The scaffold (5-mm width and 5-mm length) composed of random poly(L-lactide-co-glycolide) (PLGA) nanofibers was used to bridge the gap between the infraspinatus tendon and humerus in a rabbit rotator cuff injury model. (B) The scaffold composed of aligned poly(ε-caprolactone) nanofiber scaffolds was implanted at the site between the supraspinatus tendon and humerus in a rat rotator cuff injury model. (C) The biphasic nanofiber scaffold composed of an aligned PLGA nanofiber layer and a PLGA–hydroxylapatite composite nanofiber layer was inserted between the bone and the detached tendon for integrative rotator cuff repair in a rat rotator cuff injury model. (D) The poly(ε-caprolactone) nanofiber scaffold with dual gradations in fiber organization and mineral content was used to repair rotator cuff injury in a rat shoulder model by inserting the mineral end into the bone tunnel and suturing the aligned end to the tendon.
Reproduced with permission from [120–121].
Nanomedicine. 2013;8(9):1459-1481. © 2013 Future Medicine Ltd.