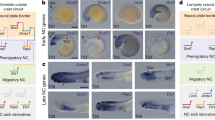
Abstract
In vertebrate embryos, Hedgehog (Hh) is expressed in some anterior basal plate domains and by notochord and floorplate cells, and ventral neural cells are patterned by the activities of Hh-regulated transcription factors. Hh signalling is antagonized by signals from the dorsal neural tube and loss of Hh leads to loss of ventral patterning as dorsal pattern expands. These mechanisms are critical for producing the neurons that implement motor responses to sensory inputs but understanding how they evolved has been hindered by lack of insight from commonly studied invertebrates where nervous system morphology and genetic mechanisms are non-conserved with vertebrates. The invertebrate chordate amphioxus, which expresses Hh in its notochord and floorplate, provides a window into the prevertebrate condition. We examined amphioxus neural development by manipulating Hh and downstream genes involved in neural pattern and cell identity. We show that Hh signalling regulates the differentiation of some neurons in amphioxus, including a subset of motor neurons. This demonstrates some conservation of mechanism between vertebrates and amphioxus. However, other aspects of neural patterning differ between the lineages. We suggest the complexity of Hh-dependent neural patterning in vertebrates evolved in a step-wise manner. Alongside other previously described regulatory changes, initial recruitment of Hh along the length of the axis occurred in an ancestor to the chordates to regulate the differentiation of a subset of neurons. This was followed, in the vertebrate lineage, by additional changes to the downstream gene regulatory network of transcription factors, giving Hh a broader role in dorsal–ventral neural patterning.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analysed during this study are in the main text, Extended Data figures and Supplementary material.
References
Gouti, M., Metzis, V. & Briscoe, J. The route to spinal cord cell types: a tale of signals and switches. Trends Genet. 31, 282–289 (2015).
Alaynick, W. A., Jessell, T. M. & Pfaff, S. L. Snapshot: spinal cord development. Cell 146, e171 (2011).
Jeong, J. H. & McMahon, A. P. Growth and pattern of the mammalian neural tube are governed by partially overlapping feedback activities of the hedgehog antagonists patched 1 and Hhip1. Development 132, 143–154 (2005).
Delile, J. et al. Single cell transcriptomics reveals spatial and temporal dynamics of gene expression in the developing mouse spinal cord. Development 146, dev173807 (2019).
Hatten, M. E. Central nervous system neuronal migration. Annu. Rev. Neurosci. 22, 511–539 (1999).
Chizhikov, V. V. & Millen, K. J. Roof plate-dependent patterning of the vertebrate dorsal central nervous system. Dev. Biol. 277, 287–295 (2005).
Chiang, C. et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383, 407–413 (1996).
Krauss, S., Concordet, J. P. & Ingham, P. W. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell 75, 1431–1444 (1993).
Uygur, A. et al. Scaling pattern to variations in size during development of the vertebrate neural tube. Dev. Cell 37, 127–135 (2016).
Kicheva, A. et al. Coordination of progenitor specification and growth in mouse and chick spinal cord. Science 345, 1254927 (2014).
Goulding, M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat. Rev. Neurosci. 10, 507–518 (2009).
Gans, C. & Northcutt, R. G. Neural crest and the origin of vertebrates—a new head. Science 220, 268–273 (1983).
Shimeld, S. M. & Holland, P. W. H. Vertebrate innovations. Proc. Natl Acad. Sci. USA 97, 4449–4452 (2000).
Denes, A. S. et al. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in Bilateria. Cell 129, 277–288 (2007).
Miyamoto, N. & Wada, H. Hemichordate neurulation and the origin of the neural tube. Nat. Commun. 4, 2713 (2013).
Doe, C. Q. Temporal patterning in the Drosophila CNS. Annu. Rev. Cell Dev. Biol. 33, 219–240 (2017).
Nederbragt, A. J., van Loon, A. E. & Dictus, W. J. Evolutionary biology: hedgehog crosses the snail’s midline. Nature 417, 811–812 (2002).
Brunet, T., Lauri, A. & Arendt, D. Did the notochord evolve from an ancient axial muscle? The axochord hypothesis. Bioessays 37, 836–850 (2015).
Holland, L. Z. Genomics, evolution and development of amphioxus and tunicates: the Goldilocks principle. J. Exp. Zool. B Mol. Dev. Evol. 324, 342–352 (2015).
Holland, L. Z. The origin and evolution of chordate nervous systems. Phil. Trans. R. Soc. Lond. B 370, 20150048 (2015).
Albuixech-Crespo, B. et al. Molecular regionalization of the developing amphioxus neural tube challenges major partitions of the vertebrate brain. PLoS Biol. 15, e2001573 (2017).
Ferran, J. L. & Puelles, L. Lessons from Amphioxus bauplan about origin of cranial nerves of vertebrates that innervates extrinsic eye muscles. Anat. Rec. (Hoboken) 302, 452–462 (2019).
Shimeld, S. M. The evolution of the hedgehog gene family in chordates: insights from amphioxus hedgehog. Dev. Genes Evol. 209, 40–47 (1999).
Hu, G. W., Li, G., Wang, H. & Wang, Y. Q. Hedgehog participates in the establishment of left–right asymmetry during amphioxus development by controlling Cerberus expression. Development 144, 4694–4703 (2017).
Lorberbaum, D. S. et al. An ancient yet flexible cis-regulatory architecture allows localized Hedgehog tuning by patched/Ptch1. eLife 5, e13550 (2016).
Holland, L. Z., Schubert, M., Kozmik, Z. & Holland, N. D. AmphiPax3/7, an amphioxus paired box gene: insights into chordate myogenesis, neurogenesis, and the possible evolutionary precursor of definitive vertebrate neural crest. Evol. Dev. 1, 153–165 (1999).
Holland, L. Z., Venkatesh, T. V., Gorlin, A., Bodmer, R. & Holland, N. D. Characterization and developmental expression of AmphiNk2-2, an NK2 class homeobox gene from Amphioxus (Phylum Chordata; Subphylum Cephalochordata). Dev. Genes Evol. 208, 100–105 (1998).
Park, H. C., Shin, J. & Appel, B. Spatial and temporal regulation of ventral spinal cord precursor specification by Hedgehog signaling. Development 131, 5959–5969 (2004).
Lauderdale, J. D. et al. Regulation of netrin-1a expression by hedgehog proteins. Mol. Cell Neurosci. 11, 194–205 (1998).
Dasen, J. S. Evolution of locomotor rhythms. Trends Neurosci. 41, 648–651 (2018).
Nomaksteinsky, M. et al. Ancient origin of somatic and visceral neurons. BMC Biol. 11, 53 (2013).
Lacalli, T. C. & Kelly, S. J. Somatic motoneurones in amphioxus larvae: cell types, cell position and innervation patterns. Acta Zool. 80, 113–124 (1999).
Bone, Q. The organization of the atrial nervous system of amphioxus (Branchiostoma lanceolatum (Pallas)). Phil. Trans. R. Soc. Lond. B 243, 241–269 (1961).
Candiani, S., Moronti, L., Ramoino, P., Schubert, M. & Pestarino, M. A neurochemical map of the developing amphioxus nervous system. BMC Neurosci. 13, 59 (2012).
Lacalli, T. & Candiani, S. Locomotory control in amphioxus larvae: new insights from neurotransmitter data. Evodevo 8, 4 (2017).
Jackman, W. R., Langeland, J. A. & Kimmel, C. B. islet reveals segmentation in the amphioxus hindbrain homolog. Dev. Biol. 220, 16–26 (2000).
Ferrier, D. E. K., Brooke, N. M., Panopoulou, G. & Holland, P. W. H. The Mnx homeobox gene class defined by HB9, MNR2 and amphioxus AmphiMnx. Dev. Genes Evol. 211, 103–107 (2001).
Wang, Y., Zhang, P. J., Yasui, K. & Saiga, H. Expression of Bblhx3, a LIM-homeobox gene, in the development of amphioxus Branchiostoma belcheri tsingtauense. Mech. Dev. 117, 315–319 (2002).
Bardet, P. L. et al. Expression of estrogen-receptor related receptors in amphioxus and zebrafish: implications for the evolution of posterior brain segmentation at the invertebrate-to-vertebrate transition. Evol. Dev. 7, 223–233 (2005).
Ishibashi, M. & McMahon, A. P. A sonic hedgehog-dependent signaling relay regulates growth of diencephalic and mesencephalic primordia in the early mouse embryo. Development 129, 4807–4819 (2002).
Epstein, D. J., McMahon, A. P. & Joyner, A. L. Regionalization of sonic hedgehog transcription along the anteroposterior axis of the mouse central nervous system is regulated by Hnf3-dependent and -independent mechanisms. Development 126, 281–292 (1999).
Osorio, J., Mazan, S. & Retaux, S. Organisation of the lamprey (Lampetra fluviatilis) embryonic brain: insights from LIM-homeodomain, Pax and hedgehog genes. Dev. Biol. 288, 100–112 (2005).
Sugahara, F., Murakami, Y., Adachi, N. & Kuratani, S. Evolution of the regionalization and patterning of the vertebrate telencephalon: what can we learn from cyclostomes? Curr. Opin. Genet. Dev. 23, 475–483 (2013).
Pani, A. M. et al. Ancient deuterostome origins of vertebrate brain signalling centres. Nature 483, 289–294 (2012).
Takatori, N., Satou, Y. & Satoh, N. Expression of hedgehog genes in Ciona intestinalis embryos. Mech. Dev. 116, 235–238 (2002).
Harris, R., Sabatelli, L. M. & Seeger, M. A. Guidance cues at the Drosophila CNS midline: identification and characterization of two Drosophila Netrin/UNC-6 homologs. Neuron 17, 217–228 (1996).
Mitchell, K. J. et al. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron 17, 203–215 (1996).
Shimeld, S. An amphioxus netrin gene is expressed in midline structures during embryonic and larval development. Dev. Genes Evol. 210, 337–344 (2000).
Simonnet, F., Deutsch, J. & Queinnec, E. hedgehog is a segment polarity gene in a crustacean and a chelicerate. Dev. Genes Evol. 214, 537–545 (2004).
Lauri, A. et al. Development of the annelid axochord: insights into notochord evolution. Science 345, 1365–1368 (2014).
Arimoto, A. & Tagawa, K. Hedgehog expression during development and regeneration in the Hemichordate, Ptychodera flava. Zool. Sci. 32, 33–37 (2015).
Lowe, C. J. et al. Dorsoventral patterning in hemichordates: insights into early chordate evolution. PLoS Biol. 4, e291 (2006).
Hudson, C., Ba, M., Rouviere, C. & Yasuo, H. Divergent mechanisms specify chordate motoneurons: evidence from ascidians. Development 138, 1643–1652 (2011).
Lara-Ramirez, R., Perez-Gonzalez, C., Anselmi, C., Patthey, C. & Shimeld, S. M. A Notch-regulated proliferative stem cell zone in the developing spinal cord is an ancestral vertebrate trait. Development 146, dev166595 (2019).
Li, G., Shu, Z. H. & Wang, Y. Q. Year-round reproduction and induced spawning of Chinese amphioxus, Branchiostoma belcheri, in laboratory. PLoS ONE 8, e75461 (2013).
Li, G., Yang, X., Shu, Z. H., Chen, X. Y. & Wang, Y. Q. Consecutive spawnings of Chinese amphioxus, Branchiostoma belcheri, in captivity. PLoS ONE 7, e50838 (2012).
Liu, X., Li, G., Feng, J., Yang, X. & Wang, Y. Q. An efficient microinjection method for unfertilized eggs of Asian amphioxus Branchiostoma belcheri. Dev. Genes Evol. 223, 269–278 (2013).
Wang, H., Li, G. & Wang, Y. Q. Generating amphioxus Hedgehog knockout mutants and phenotype analysis. Yi Chuan 37, 1036–1043 (2015).
Li, G. et al. Mutagenesis at specific genomic loci of amphioxus Branchiostoma belcheri using TALEN method. J. Genet. Genomics 41, 215–219 (2014).
Li, G. et al. Cerberus-Nodal-Lefty-Pitx signaling cascade controls left–right asymmetry in amphioxus. Proc. Natl Acad. Sci. USA 114, 3684–3689 (2017).
Yu, J. K. & Holland, L. Z. Amphioxus whole-mount in situ hybridization. Cold Spring Harb. Protoc. 2009, pdb prot5286 (2009).
Wu, H. R. et al. Asymmetric localization of germline markers Vasa and Nanos during early development in the amphioxus Branchiostoma floridae. Dev. Biol. 353, 147–159 (2011).
Acknowledgements
We thank Z. Zuo and Y. Zhou for their help with qrtPCR. Collaboration between G.L. and S.M.S. was supported by an International Exchanges 2017 Cost Share award funded by The Royal Society (grant no. IEC\NSFC\170126) and the National Natural Science Foundation of China (grant no. 31811530298). The work is also supported by grants from the National Natural Science Foundation of China (nos. 31872186, 31672246 and 31471986).
Author information
Authors and Affiliations
Contributions
G.L., S.M.S., Q.R. and Y.W. designed experiments. Q.R., Y.Z., X.H., G.L., C.X. and G.H. conducted experiments and collected data. G.L., S.M.S., Q.R., Y.W., Y.Z., X.H., C.X., B.L., H.W. and G.H. analysed the data. S.M.S., G.L. and Q.R. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
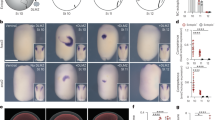
Extended Data Fig. 1 Ptch expression in Hh-/- and Smo-/- embryos.
Whole mounts with anterior to the left and the scale bars are 50 µm, with the bars in A-F also applying to A’-F’ respectively. All the embryos are in lateral view. Expression of Ptch in (a–c) control (Hh+/+ and Hh+/-) and (a’–c’) Hh-/- embryos at neurula (8 S and 14 S) and larval stages. Expression of Ptch in (d–f) control (Smo+/+ and Smo+/-) and (d’–f’) Smo-/- embryos at neurula and larval stages. 8 S: embryos with 8 somites; 14 S: embryos with 14 somites. Numbers in the bottom right corner of a panel show the number of times the phenotype was seen, out of the number of embryos of that genotype analysed.
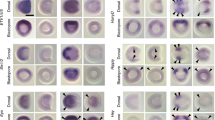
Extended Data Fig. 2 The expression of neural tube marker genes in Hh-/- and Ptch-/- embryos at 3-somite neurula stage.
Anterior is to the left and the scale bar is 50 µm, the images are in lateral views except for a, a’, e, f’, l, l’, M and M’, which are ventral views focused on the dorsal side. Expression patterns of neural tube marker gene Dlx, IrxA, IrxB, IrxC, Pax3/7, Pax4/6 and Nkx2.2 in (a–g) control (Hh+/+ and Hh+/-) and (a’–g’) Hh-/- embryos at early neurula stage. Expression patterns of neural tube marker gene Dlx, IrxA, IrxB, IrxC, Pax3/7, Pax4/6 and Nkx2.2 in (H-N) control (Ptch+/+ and Ptch+/-) and (h’–n’) Ptch-/- embryos at early neurula stage. Numbers in the bottom right corner of a panel show the number of times the phenotype was seen, out of the number of embryos of that genotype analysed.
Extended Data Fig. 3 The expression of neural tube marker genes in Hh-/- and Ptch-/- embryos at 5-somite neurula stage.
The scale bar in A is 50 μm and applies to other embryos, the scale bar in a is 20 μm and applies to other sections. Embryos are shown with anterior to the left and in lateral view except for a, a’, e, e’, h, h’, l, l’. Sections are transverse with dorsal to the top, and at the point indicated by the black line in the embryo depicted to the left of each section. Numbers in the bottom right corner of a panel show the number of times the phenotype was seen, out of the number of embryos of that genotype analysed. Embryos and sections in a row show the expression of the same gene, indicated at the far left of the row.
Extended Data Fig. 4 The expression of neural tube marker genes in Hh-/- and Ptch-/- embryos at 14-somite neurula stage.
The scale bar in A is 50 μm and applies to other embryos, the scale bar in a is 20 μm and applies to other sections. Embryos are shown with anterior to the left and in lateral view except for a, a’, e, e’, h, h’, l, l’. Sections are transverse with dorsal to the top, and at the point indicated by the black line in the embryo depicted to the left of each section. Numbers in the bottom right corner of a panel show the number of times the phenotype was seen, out of the number of embryos of that genotype analysed. Embryos and sections in a row show the expression of the same gene, indicated at the far left of the row.
Extended Data Fig. 5 Double in situ hybridization of Mnxa and OligA.
Confocal images of WT amphioxus at 11 somites stage in (a–c) lateral views and in (a’–c’) ventral views focused on the dorsal side. (a) Merge of (b) Mnxa+ neurons (green) and (c) OligA+ neurons (red), the white arrows in A mark the coexpresssion positions of Mnxa and OligA (yellow). (a’) Merge of (b’) Mnxa+ neurons (green) and (c’) OligA+ neurons (red), the white arrows in A’ mark the coexpresssion positions of Mnxa and OligA (yellow) in anterior neural tube. Whole mounts are shown with anterior to the left and the scale bar is 50 µm.
Extended Data Fig. 6 Nkx6, OligA or Mnxa mRNA injection could not restore the motor neuron defects to normal in Hh-/- amphioxus.
Whole mounts with anterior to the left and scale bar is 50 µm. All the embryos are in lateral views and at 9 somites stage (middle neurula stage). The expression of Mnxa in the neural tube of (a) control (Hh+/+ and Hh+/-) and (b) Hh-/- embryos. (c, d) The expression of Mnxa in embryos injected with Nkx6 mRNA. (e, f) The expression of Mnxa in the neural tube of embryos injected with OligA mRNA. (g, h) The expression of Mnxa in the neural tube of embryos injected with Nkx6 and OligA mRNA. The expression of Lhx3 in the neural tube of (i) control (Hh+/+ and Hh+/-) and (j) Hh-/- embryos. (k, l) The expression of Lhx3 in the neural tube of embryos injected with Mnxa mRNA. (m, n) The expression of Lhx3 in the neural tube of embryos injected with OligA, Nkx6 and Mnxa mRNA. Numbers in the bottom right corner of a panel show the number of times the phenotype depicted was confirmed, out of the total number of embryos of that genotype on which the experiment was performed.
Extended Data Fig. 7 OligA, Nkx6, Lhx3, Mnxa, Islet and Vacht expression in OligA-/- embryos.
Whole mounts with anterior to the left and the scale bar is 50 µm. Ventral views focused on the dorsal side except for e and e’, which are lateral views. (a, a’, d and d’) The expression of OligA and Mnxa in control (OligA+/+ and OligA+/-) and OligA-/- embryos at 11 somites stage. (b, c’ and e, f’) The expression of Nkx6, Lhx3, Islet and Vacht in control (OligA+/+ and OligA+/-) and OligA-/- embryos at 14 somites stage. Numbers in the bottom right corner of a panel show the number of times the phenotype was seen, out of the number of embryos of that genotype analysed.
Extended Data Fig. 8 The expression of Lhx and Islet in Mnxa-/- amphioxus embryos at 14 somites stage.
Top panels show digestion products from PCR across the mutated region: mutation abolishes the restriction site, so the higher band (uncut) indicates presence of the mutated allele, the lower band (cut) the wild-type allele. Lower panels show Lhx and Islet expression in embryos analysed. Embryo numbers and genotypes are shown on the top left corner of each image, with -/- highlighted in red. Whole mounts with anterior to the left. Scale bars are 50 µm. The embryos stained with Lhx probes are all in ventral view, focused on the dorsal side, except number #4 embryo which is in lateral view; the embryos stained with Islet probes are all in lateral view.
Extended Data Fig. 9 The expression of Vglut and Vgat in Hh-/- amphioxus embryos at 14 somites stage.
Whole mounts with anterior to the left. Scale bar is 50 µm. The embryos are all in ventral view, focused on the dorsal side. Hh+/+;+/- and Hh-/- embryos are distinguished according to symmetry of somites as indicated by red dotted lines (oblique lines show asymmetrical arrangement of somites in Hh+/+ and Hh+/- embryos, vertical lines show symmetrical arrangement of somites in Hh-/- embryos).
Extended Data Fig. 10 Overlapping expression of Err, Vacht and Mnxa revealed by double in suit hybridization.
Whole mounts with anterior to the left and the scale bars are 50 µm. (a) Merge of (b) Err+ neurons (black) and (c) Vacht+ neurons (red) in WT amphioxus neural tube at 14 somites stage in ventral view, focused on the dorsal side. (d) Merge of (e) Mnxa+ neurons (red) and (f) Vacht+ neurons (Green) in WT amphioxus neural tube at 11 somites stage in lateral views, the white arrows in D mark the coexpresssion positions of Mnxa and Vacht in amphioxus neural tube. (g) Merge of (h) Mnxa+ neurons (red) and (I) Vacht+ neurons (Green) in WT amphioxus neural tube at 11 somites stage in ventral view, focused on the dorsal side, the white arrows in G mark the coexpresssion positions of Mnxa and Vacht in amphioxus neural tube. Panels a–c are each collages of two separate photographs, with the boundary between the images identified in the figure.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6 and Tables 1–3.
Rights and permissions
About this article
Cite this article
Ren, Q., Zhong, Y., Huang, X. et al. Step-wise evolution of neural patterning by Hedgehog signalling in chordates. Nat Ecol Evol 4, 1247–1255 (2020). https://doi.org/10.1038/s41559-020-1248-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-020-1248-9
This article is cited by
-
Amphioxus Gli knockout disrupts the development of left–right asymmetry but has limited impact on neural patterning
Marine Life Science & Technology (2023)
-
Pitx controls amphioxus asymmetric morphogenesis by promoting left-side development and repressing right-side formation
BMC Biology (2021)