Neurotechnology devices are opening doors to new therapies in unmet medical markets.
July 1, 2007
NEUROTECHNOLOGY
|
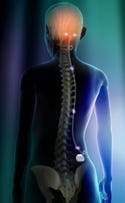
Neuromodulation sends electrical pulses to the brain or central nervous system. Illustration by David Dunston, Zygote Media Group. |
Traditional drug therapy failed them. Pat, whose doctors initially prescribed 10 mg a day of a painkiller for her chronic back pain still suffers three years later, even at 300 mg a day. Bob found relief from his depression for a time—until the medication could no longer hold back the feelings of hopelessness and anxiety.
For Ruth, it isn't medication, but physical therapy that has proved ineffective. After a stroke, Ruth is paralyzed on the left side of her body. She may regain some movement with physical therapy, but she can recover only minimal function. Like most stroke victims, she will have to adapt to her disabilities.
For Pat, Bob, and Ruth, hope may come from technology. A handful of neurotechnology companies have been quietly working to change the lives of these patients. New therapies—in particular, neuromodulation—are being developed, reaping the results of years of research that has provided a better understanding of the brain and the central nervous system.
|
In 2006, neurotechnology products generated more than $120 billion in revenue, with the market seeing 10% growth over the previous year. Despite its already large size, the neurotechnology sector is poised for large growth with several factors driving this demand, says Zack Lynch. Lynch is executive director of the Neurotechnology Industry Organization (NIO). Primary among these, he says, is that neurotechnology companies address brain-related injuries and illnesses, the largest unmet medical market.
In 2007, neuromodulation is estimated to be a $1.8 billion market, adds Mona Patel, vice president of marketing for the pain management division of Boston Scientific. This market includes spinal cord stimulators, cochlear implants, deep-brain stimulators, drug pumps, and vagus nerve stimulators. “These devices have helped more than 300,000 people regain some level of normalcy in their lives,” she says.
Neurotechnology is a pretty broad category, notes John Bowers, CEO of Northstar Neuroscience. “There are great diagnostic and imaging tools that have been developed over recent years, and there are pharmacologic therapies,” he says. Then there are implantable, electrical therapies that are themselves inherent to the therapy. “That's what's most exciting: when a device can actually be the therapy or be integral to the therapy itself. When you're able to accomplish that, that's the Holy Grail of medical technology. That's why we're interested in that area,” he says.
As early as 10 years ago, devices were beginning to be approved for the treatment of epilepsy, and a host of devices are now in pivotal trials and feasibility studies for drug- resistant depression, stroke recovery, and tinnitus, just to name a few.
|
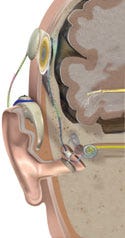
One of the earliest uses of neurotechnology was for the development of cochlear implants. Image courtesy of Advanced Bionics (Valencia, CA), a Boston Scientific company. |
An Electrical Approach
“The central nervous system is a miraculous symphony of chemistry and electricity that is in almost perfect balance in healthy individuals,” says Christopher Chavez, president of St. Jude Medical's neuromodulation division. When the central nervous system is out of balance because of trauma, disease, aging, or other causes, people can experience hard-to-manage chronic pain and diseases including chronic depression, Parkinson's, Alzheimer's, and many others, he says.
|
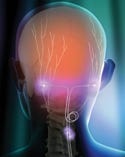
The Genesis neurostimulation system is being tested in clinical research as a therapy for helping patients with chronic migraine headache. Genesis is an investigational system that sends mild electrical pulses to the occipital nerves in the back of the head from a device implanted in the buttock or chest. Illustration at bottom of facing page and at left provided by Advanced Neuromodulation Systems (Plano, TX), the neuromodulation business of St. Jude Medical. |
Advances in technologies such as neuromodulation are for the first time making it possible to address these conditions. “I think about the millionsand millions of people who suffer from some type of neurological disorder or disease that really isnot adequately treated today,” says Bowers. “If you look atthe medical technology industry and all of the advanceswe've made over the last 40 years for cardiovascular therapies,for example, there are still huge opportunities for developingeffective therapies for neurological disorders.”
Those in this business are passionate about its future. Patel notes that although relatively unknown, the science of neuromodulation has been around for decades. Recent advances in the underlying science and in technology are making it possible for neurotechnology devices to change the practice ofmedicine today.
“This science has already allowed deaf people to hear. Tomorrow brings the promise of making the blind able to see,” she says. “Scientists and engineers around the world are exploring how to modulate the nervous system to address such common ailments as migraines, depression, obesity, Parkinson's, and epilepsy. Therefore, if you look across the field of medicine, there are only a few areas that hold as much promise in the nextdecade as neuromodulation.”
|
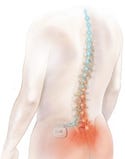
The Eon spinal cord stimulation system is approved for the treatment of chronic pain of the trunk and limbs and failed back surgery syndrome. A device implanted in the buttock sends mild electrical pulses to leads located near the spinal cord. Illustration courtesy of Advanced Neuromodulation Systems (Plano, TX), the neuromodulation business of St. Jude Medical. |
Throughout history, humanity has explored multiple modalities to treat chronic diseases, says Chavez. “Recently, modern medicine has significantly advanced the use of systemic drugs and surgery to treat such conditions,” he says, “and more advances are on the horizon.” Even so, many chronic diseases remain undertreated because they are refractory, or resistant, to existing therapies. The new therapeutic strategies now being developed are needed to address these diseases, especially those related to neurological disordersand an aging population.
“It's already happening in some areas,” says Dan Moore, CEO of Cyberonics. Several devices, including the company's vagus nerve stimulation (VNS) device, are enjoying broad adoption and acceptance by physicians. In most cases, Moore says, neurotechnology is requiring physiciansto change the way they treat patients.
|
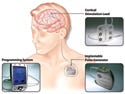
(click to enlarge) The VNS Therapy System uses asurgically implanted medical devicethat delivers electrical pulsedsignals to the vagus nerve in theleft side of the neck. Illustrationcourtesy of Cyberonics (Houston). |
“Most neurological disorders are treated with pharmaceuticals, but the brain is an electrical organ, so it's logical to apply an electrical approach to treating neurological disorders.”
According to Chavez, advances in microelectronics and in the understanding of neurophysiology have created an opportunity to treat the central nervous system's electrical-chemical system using neuromodulation. He says that the delivery of microdoses of electricity or drugs to specific neural targets offers tremendous potential to address a significant and expanding list of clinical applications.
The Gold Standard
Neuromodulation is one of the fastest-growing emerging technologies and is one of the most promising, says Patel. The market for neuromodulation devices, she says, is estimated to grow 20% a year and will likely maintain that growth for well over a decade. “The fact that neuromodulation can address a variety of disease states that have very few—or often, much more invasive—options makes this an ideal way to treat these conditions.”
Chavez also credits advances in technology and an increased understanding of the central nervous system for the growth of the industry. “These advances will accelerate innovative and entrepreneurial activity in the neuromodulation field,” he says. “The market for neuromodulation is approaching $2 billion worldwide and is likely to grow as more indications for the modality are developed. Neuromodulation is already helping thousands of people and promises to help millions more in the future.”
|
“Neuromodulation is likely to become the gold standard of treating many chronic diseases.” — Christopher Chavez |
In terms of specific clinical indications, Bowers says the biggest advances are being seen in spinal cord stimulation for pain, deep-brain stimulation for movement disorders, and VNS for epilepsy. “No single therapy will solve all chronic medical problems,” says Chavez. “We must explore multiple strategies and modalities to address the many difficult-to-treat conditions that destroy the lives of so many people.”
In a world where change and innovation have become predictable constants, says Chavez, those who work in neuromodulation will embrace new knowledge and technology to make products smaller, smarter, and more affordable.“An important strategy for the future will be to invest inclinical studies that demonstrate the safety, efficacy, andvalue of such products, then to make sure that patients, insurers,and physicians have information to select the righttechnology at the right time for each patient. Neuromodulationis likely to become an important therapeutic optionor even the gold standard for treating many chronic diseases,”says Chavez.
Moore agrees. “Neurostimulation devices are the next frontier in medicine,” he says. “Much like cardiac pacemakers some decades back, the neurostimulation market is burgeoning as it addresses huge patient populations in epilepsy, depression, stroke, anxiety disorders, Parkinson's andothers with tremendous unmet needs.”
Understanding the Science
A better understanding of the underlying science has been key to the advances in neuromodulation, says Bowers, noting that just 20 years ago, the idea that the brain could change was not widely discussed or accepted. “If you had a brain injury or some type of neurological disorder, it wasthought of as irreversible.
“Over the years, scientists have learned that the brain is plastic. Through neuroplasticity, the brain changes over time. For example, it changes in response to an injury such as a stroke or a traumatic brain injury,” he says. “Although the cells in the area of the brain that was destroyed by a stroke aren't coming back, the brain forms alternate neural connections that attempt to take over the function for the area that was destroyed.” That concept wasn't widely understood 20 years ago. Today it is, so the idea that you can take a targeted device therapy through electrical stimulation and help enhance that process—that's what's new, says Bowers.
The neurotechnology industry has also taken some base technologies that have proven effective in other areas—such as cardiac applications—to find novel ways to deliver therapies elsewhere in the body. The result has been devices that provide electrical stimulation of various areas to the cerebral cortex.
|
The Eon spinal cord stimulation system. Illustration courtesy of Advanced Neuromodulation Systems (Plano, TX), the neuromodulation business of St. Jude Medical. |
Certainly for spinal cord stimulation, the push for improved patient outcomes has been a major driver for the market, notes Patel. She also points to significant advances in technology such as the introduction of rechargeable devices, which have allowed newer devices to offer a greater range of parameters. Devices now have pulse widths as well as leads that can deliver an electric drug to the target site with greater precision. “Clinicians can now treat patients that just three years ago would not have been deemed candidates for spinal cord stimulation, or SCS, devices.”
|
“There are few areas that hold as much promise in the next decade as neuromodulation.” — Mona Patel |
Advanced Bionics, which was purchased by Boston Scientific in 2004, introduced a system that uses multiple independent current sources to relieve pain, a key advance in this field. This system is the equivalent of having 16 implantable pulse generators or batteries of the older systems, explains Patel. “This allows it to better target nerves and maintain therapy over time.”
The demographics are favorable for neuromodulation therapy. “The population is aging, and many of the disease states that neuromodulation can address occur later in life. And baby boomers have higher expectations of the quality of life in later years,” says Patel. “The average age for someone to receive an SCS implant is 55. Therefore, many people are in their prime when they are severely disabled. Just like other areas of medicine, the prospect of the Internet as a way to educate patients is promising, and notably, 70% of Advanced Bionics customers use the Internet regularly. The fact that this target population is Internet savvy, and will become even more so in the future, only further supports growth of this therapy.”
Although neuromodulation is just hitting its stride now, it has already made some significant inroads as a therapy of choice for some disorders. This July marks the 10th anniversary of FDA approval of Cyberonics's VNS Therapy for pharmacoresistant epilepsy, says Moore. “More than 45,000 patients worldwide have been implanted with VNS Therapy for both epilepsy and treatment-resistant depression combined. One of the biggest drivers in the adoption of neurotechnology therapies is the growing body of data and the adoption rate among physicians,” he says. “There is a greater understanding of the types of patients for whom nonpharmacological options are appropriate.”
A study of depression found that one-third of patients with depression are not helped by antidepressant medications. The study, “Results for Sequenced Treatment Alternatives to Relieve Depression, or STAR*D,” was funded by the National Institute of Mental Health. The researchers noted that other options, including VNS Therapy, should beevaluated for those patients.
Within many of the disease states targeted by neuromodulation, a significant market opportunity is for those patients that are drug refractory, says Patel. She says that addressing these patients will be the priority for clinicians.“There are thousands of patients who don't have any solutionstoday. The one clear area of differentiation is the factthat drugs have a systemic effect on the patient, whereasneuromodulation has the ability to target the specific neuralnetworks or organ.”
|
“There are still huge opportunities for developing effective therapies for neurological disorders.” — John Bowers |
In most cases, says Moore, neuromodulation devices have been approved as adjunctive therapies or to treat patients who have tried many pharmaceuticals without getting relief. Many patients are treated appropriately and successfully with pharmaceutical therapy. The patients who could most benefit from devices like VNS Therapy are those for whom medications do not help to reduce symptoms or for whom the side effects of those medications becomeintolerable.
Neuromodulation has been effective in its ability to apply targeted stimulation to yield a clinical benefit, says Bowers. Rather than taking a drug systemically, it provides direct stimulation. “For the stroke indication, currently there are no effective therapies. There are no pharmacology therapiesthat help motor recovery or motor function.”
|
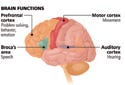
(click to enlarge) Stimulation is delivered to the appropriate site of the brain depending on the disorder being treated. Northstar Neuroscience is investigating the safety and efficacy of cortical stimulation for patients with post-stroke speech and motor deficits, major depression disorder, and tinnitus. Image courtesy of Northstar Neuro science (Seattle). |
Northstar Neuroscience has a feasibility study under way designed to treat depression. Bowers says the existing therapies, of which there are many, do not adequately meet the needs of millions of people. “Physicians, patients, and families are really looking for results. Tinnitus is another disorder for which we have a feasibility study. No effectivetherapies exist for chronic sufferers of that condition.”
The Regulatory and Reimbursement Landscape
“Adoption of neurotechnology devices takes time,” says Moore. “Just as the treating physicians are required to change the way they practice medicine, the regulatory bodies are adapting in order to be able to evaluate these new technologies and new approaches to treating neurological disorders,” he says. FDA has approved VNS Therapy for two indications. In addition, the agency is reviewing deep-brain stimulation devices for a variety of indications. “This shows that FDA recognizes the progress being made in neurotechnology,” explains Moore.“Nonetheless, more progress is needed in establishing thestandards for evaluating the safety and effectiveness ofthese new approaches to treating illness, because the clinicalevaluation of neurotechnology devices provides someunique challenges.” As an illustration, he says, determiningthe appropriate dose of the treatment may be morecomplex when using a device than it is when using a drug.“For example, with VNS Therapy, there are five differentparameters that a physician can alter when adjusting thedose of stimulation a patient will receive. This in turn resultsin an enormous number of different potential combinationsof dose settings.”
Bowers notes that implantable device therapies take about a year to move through FDA's approval process. “If you're doing a premarket approval (PMA) supplement, it can go faster, but if you look at something that truly is a new therapy, a year is about right.” He adds, “When therapies are new, you are breaking new ground in terms of what are appropriate outcome measures and making sure that any safety data are captured and reviewed appropriately. It's really important for the agency as well as for all of us who are in the business of developing new therapies to have really good communication with FDA. Communication is important not just as you go through review of your PMA, but also during the months or, in our case, years of work leading up to filing the original investigational device exemption (IDE) for the trial. This is essential so that you have a common understanding of outcome measures and data analysis.”
CMS recently denied coverage of Cyberonics's VNS Therapy for treatment-resistant depression (TRD), despite overwhelmingly favorable comments. But, in general, CMS has been fairly receptive to providing coverage for neuromodulation therapies.
“Several studies are under way to help us learn more about VNS Therapy for TRD, and we will share the growing body of evidence with CMS and other insurers as it becomes available,” says Moore. “Since [FDA] approval of VNS Therapy for TRD, more than 300 payers have provided coverage for more than 3000 patients on a case-by-case basis.”
Bowers say that CMS is sending an important message that coverage is about data. “It's about having well-run clinical trials that have a meaningful clinical outcome—one that can be understood and that shows a consistent and clear benefit for an appropriate group of patients,” he says. For example, he says, CMS routinely provides coverage for VNS for epilepsy.“We have watched and learned from [CMS's denial of VNSfor depression] as we design our trials. As we put together thedata and information package with the healthcare providers,we'll be able to move forward. If you look at reimbursementbroadly for neurostimulation, it's a positive. In general, thereare codes that exist today so manufacturers don't have to gothrough the time-consuming steps of getting codes.”
Patel points to CMS's recognition of the advances of having a rechargeable system and allowed for a pass-through code in 2006. “This additional reimbursement for rechargeable SCS systems was a key factor in the market taking off,” says Patel.
|
(click to enlarge) Northstar's Renova-ST Cortical Stimulation System is an investigational device designed to enhance recovery of stroke patients who suffer from Broca's aphasia. Image courtesy of Northstar Neuroscience (Seattle). |
Bowers adds that the payments under the codes available are at least reasonable enough so that there is not a dis - incentive for institutions to offer the therapies. And then, he stresses, any other new therapy must get coverage under those codes. “To do that, you have to have a really strong, robust clinical data set,” he says.
Neurotechnology in Practice
Clinicians have been responsive to advances that allow them to offer a solution for a patient who has been suffering for years, says Patel. With advances in technology resulting in improved outcomes, she says that more clinicians are interested in becoming experts with neuromodulation devices. “We have had more than 1000 physicians trained in cadaver courses since the introduction of the Advanced Bionics system. More and more physicians want to be able to have this therapy among those that they can offer their patients,”she says.
“Neuromodulation is changing the practice of medicine,” notes Bowers. “Clinicians are very hopeful and interested in these therapies because they have patients whose ailments aren't being adequately addressed today, and they'd like to have solutions to offer them.” For example, he points to the millions of people in the United States who have had a stroke and have some level of paralysis of their hand and arm. Right now, nothing has proven to be effective for motor recovery in the years following a stroke. “So that's one example where there are certainly a lot of physicians, clinicians, and therapists who would like to be able to offer hope to those patients via an effective therapy. So they're very interested in what we're doing,” he says.
As they are with any therapy, though, physicians want clinical data, says Bowers. “They want you to be able to show to them validated outcome measures that you're having an effect with a well-run study and that the effect really makes a difference in that patient's life.”
Physicians are excited about the potential of neurostimulation, says Moore. “We see this in the adoption of VNS Therapy for approved indications such as epilepsy and TRD, as well as in the interest in research in other potential therapeutic indications. At a recent meeting of the American Psychiatric Association in San Diego, Moore says the consensus among psychiatrists prescribing VNS Therapy was that it provides measurable and effective relief to their patients.
Conclusion
Brain-related illnesses generate more healthcare costs than any other therapeutic area—$1.1 trillion annually in the United States, says NIO's Lynch. With new tools to better understand the brain, though, neurotechnology companies are making great strides to develop products to address these illnesses. In many cases, advances in technologies such as neuromodulation are for the first time making it possible to address conditions that are drug resistant.
With market growth moving into double digits, neurotechnology devices have the potential to change the practice of medicine for a number of serious age-related diseases and conditions, including Parkinson's, Alzheimer's, and stroke.
Copyright ©2007 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like