Insights into the mechanism of ultraviolet light damage and cancer lesions may contribute to anticancer therapy
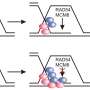
![RFWD3- and Polκ-mediated DNA damage tolerance pathways are independent of each other, but both are dependent on PCNA modifications at K164. (A) PCNA[WT] and [KR] cells were transfected with siRFWD3#1, siPolκ#2, or siNTC#2. Cells were exposed to illudin S for 4 d and analyzed by MTS assay. Data are represented as means ± SD of n = 4 independent experiments. *P < 0.05 versus siNTC#2; #, not significant versus siNTC#2. (B) Whole-cell lysates from the cells using (A) were prepared and analyzed by immunoblotting using anti-RFWD3, anti-Polκ, and anti-Lamin B1 antibodies. The arrowhead shows the RFWD3 signal. (C) HeLaS3 and HeLaS3 Polκ KO cells were transfected with siRFWD3#1 or siNTC#2 and exposed to illudin S for 4 d. HeLaS3 PCNA[WT] or PCNA[KR] cells were exposed to illudin S for 4 d. Cellular survival was evaluated by MTS assay. Data are represented as means ± SD of n = 4 (HeLaS3+siNTC#2, HeLaS3+siRFWD3#2, and HeLaS3 Polκ KO+siNTC#2) or n = 3 (HeLaS3 Polκ KO+siRFWD3#1, HeLaS3 PCNA[WT], and PCNA[KR]) independent experiments. Statistical significance was evaluated by two-tailed t test. Credit: Life Science Alliance (2022). DOI: 10.26508/lsa.202201584 Insights into the mechanism of ultraviolet light damage and cancer lesions may contribute to anticancer therapy](https://scx1.b-cdn.net/csz/news/800a/2022/insights-into-the-mech.jpg)
A team led by researchers from Nagoya University in Japan has discovered new pathways that cells use to repair themselves following exposure to ultraviolet (UV) light, and a new agent involved in these pathways known as RFWD3. This could lead to future treatments for people with photosensitive diseases and prompt the development of better anticancer medicines. "We believe our findings provide a new perspective to the field of DNA damage response and also to cell biology," said lead author, Chikahide Masutani. They published their research in the journal Life Science Alliance.
Similar to how our body gets cuts and abrasions as we go through life, DNA can also sustain small bits of cumulative damage. One common form of such damage is a DNA lesion. Exposure to UV light, for instance, can cause a section of DNA to contain a damaged site or alter one of its pairs. Put another way, we can think of DNA like a written sentence. In a sentence, a DNA lesion would be like a misprinted word, making it unreadable. In the same way that printing a run of books including misprinted words makes all the books illegible, a DNA lesion might make the entire genome unstable or cause permanent mutations as it is copied. These lesions are important because they are associated with many forms of cancer.
To counteract the effects of a DNA lesion, human bodies have multiple DNA damage tolerance pathways. These pathways enable replication even in the presence of lesions. Accordingly, biologists have sought to identify the factors involved in unidentified DNA damage tolerance mechanisms.
In particular, one such factor is proliferating cell nuclear antigen (PCNA). PCNA activates trans-lesion DNA synthesis, which repairs lesions using the enzyme DNA polymerase eta (Pol-eta). While this pathway is interesting because it offers cells resistance to UV irradiation and some DNA-damaging agents, other pathways may also be important, especially those pathways independent of Pol-eta.
A group of researchers from Nagoya University, led by Rie Kanao and Chikahide Masutani from the Research Institute of Environmental Medicine, have discovered new agents by intentionally creating lesions using illudin S, a mushroom toxin, and its derivative irofulven. The researchers found cells without PCNA modification were sensitive to these compounds, causing lesions. On the other hand, those lacking Pol-eta were not. Therefore, they could analyze the PCNA modification-dependent pathway of lesion repair independently of the Pol-eta pathway.
Kanao and Masutani identified that the agents in the PCNA modification-dependent pathway include RFWD3, a protein-coding gene. RFWD3 is common to both pathways, therefore this suggests that the two major branches of the pathway are Pol-eta and RFWD3 for lesions caused by UV light, and polymerase kappa and RFWD3 for those caused by illudin S. "It may be a general feature that different DNA polymerases are employed depending on the type of DNA lesion," explained Masutani. "Our findings suggest that RFWD3 contributes to PCNA modification-dependent DNA damage tolerance. This is the first description of the involvement of RFWD3 in UV-survival in human cells."
A promising use of this research is for anticancer treatment because it may become possible to inhibit DNA damage tolerance pathways. Although DNA damage tolerance is supposed to help repair lesions, cancers sometimes use the agents to help them tolerate DNA-damaging anti-cancer drugs. "We believe that the research can contribute to cancer therapy," Masutani said. "There is increasing evidence that by inhibiting DNA damage tolerance pathways, we can sensitize cancer cells to conventional chemotherapeutic agents. More research into novel therapeutics in this area could eventually lead to the development of a new class of cancer therapeutic agents that enhance response to treatment using conventional chemotherapy."
More information: Rie Kanao et al, RFWD3 and translesion DNA polymerases contribute to PCNA modification–dependent DNA damage tolerance, Life Science Alliance (2022). DOI: 10.26508/lsa.202201584
Provided by Nagoya University