The COVID-19 pandemic has been met with an unparalleled response by the global scientific community and its stakeholders, who have developed vaccines and therapeutic antibodies in an unprecedented timescale. These new therapies for preventing and treating COVID-19 have altered the course of the pandemic to an extent that has never before been seen for an infectious disease in terms of the numbers of lives saved, with vaccines alone being estimated to have helped to save 19.8 million lives within their first year of deployment1 and therapeutic antibodies providing additional protection to the most vulnerable populations2.
As was recently outlined by the National Institute of Allergy and Infectious Diseases (NIAID) in their pandemic-preparedness plan (P3), research into new technologies is a cornerstone of future pandemic-prevention strategies3. To overcome the ongoing challenges posed by COVID-19 and to minimize the impact of future pandemics, AstraZeneca is researching new technologies to support the rapid development of novel vaccines and long-acting monoclonal antibodies (LAABs) for vulnerable populations.
COMPLEMENTARY STRATEGIES TO PROTECT ALL
At AstraZeneca, our approach to tackling the most challenging infectious diseases and future pandemics encompasses two complementary strategies — active and passive immunization — so that ‘no one will be left behind’ (Fig. 1).
Active immunization is the generation of B-cell-mediated antibody and T-cell-mediated cellular immune responses to antigens from pathogens through vaccination. Vaccines are critical to disease prevention and infection control at both the individual and the population level via herd immunity.
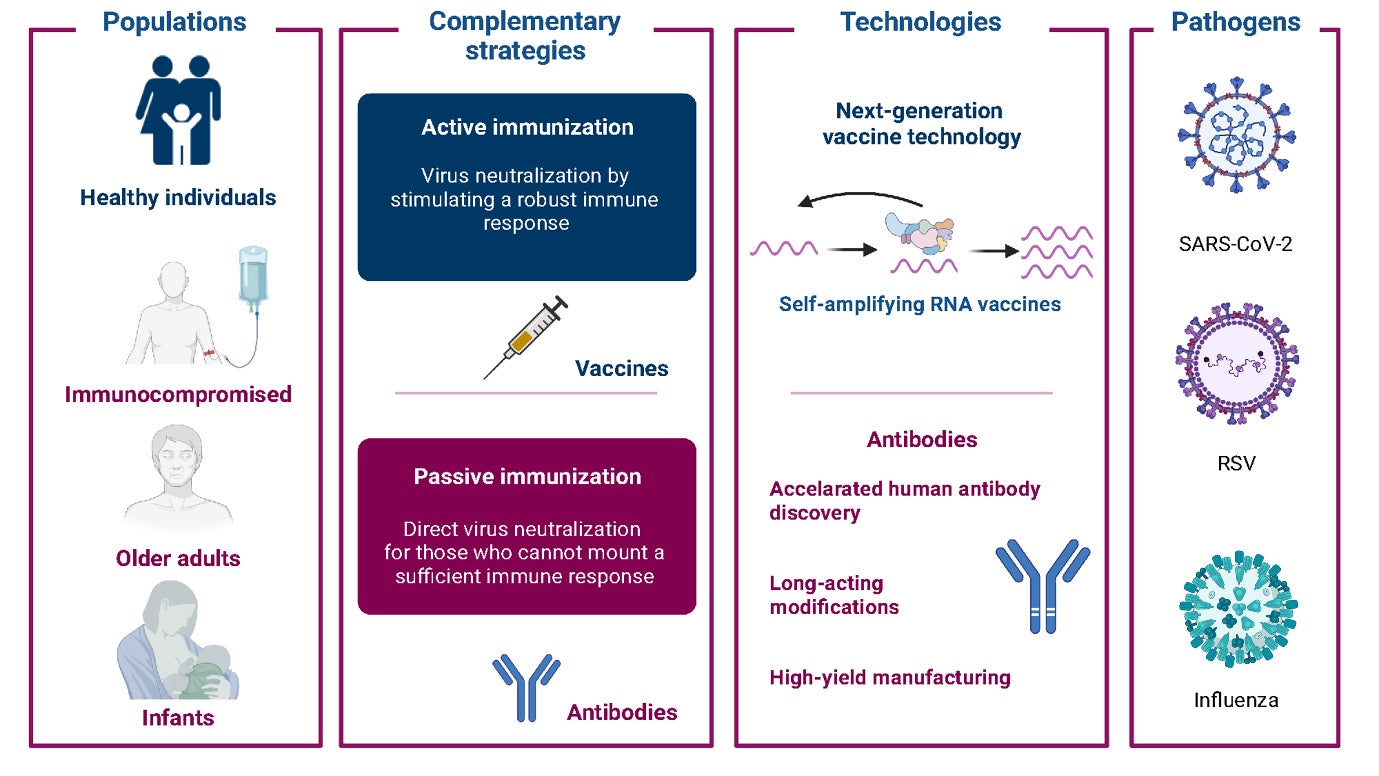
coronavirus 2 (SARS-CoV-2), respiratory syncytial virus (RSV), influenza and pathogens with pandemic potential.
However, other strategies are needed to protect those unable to fully generate an immune response following vaccination, such as individuals who are immunocompromised following organ transplantation or are receiving treatment for certain cancers or immunosuppressive therapies. The administration of exogenous antibodies by injection or intravenous infusion can provide additional protection for vulnerable individuals — a process known as passive immunization or immunoprophylaxis. Examples of passive immunization include the transfer of antibodies from mother to infant via the placenta during pregnancy or via breast milk, and the clinical administration of monoclonal antibodies (mAbs).
TRANSFORMING VACCINE DEVELOPMENT
Investment in novel vaccinedevelopment platforms that are adaptable, reliable, and scalable is necessary to ensure that sufficient doses can be produced to rapidly respond in a pandemic setting. The need for this flexibility has been exemplified by the emergence of severe acute respiratory syndrome coronavirus 2 (SARSCoV- 2) variants-of-concern that can be more transmissible and/or can evade antibodies elicited by the currently authorized COVID-19 vaccines, thereby causing an increase in breakthrough infections. Innovative approaches to vaccine development are also needed to develop pan-variant vaccines that could provide protection against multiple viral variants. AstraZeneca is addressing these types of challenges by researching self-amplifying RNA (saRNA) and novel antigendesign technologies.
saRNA is a novel vaccine platform that has been observed to elicit robust immune responses and could play a vital role in future pandemic responses due to the speed and scalability of its production. saRNA vaccines are delivered by lipid nanoparticles and have the same chemical properties as messenger RNA (mRNA)- based vaccines4. However, while mRNA vaccines contain the RNA genetic code for a single antigen, saRNA vaccines comprise the RNA of their target antigen and four additional alphavirus proteins that form the RNA-dependent replicase. This additional component allows for more target antigen mRNA to be produced within a vaccine-recipient’s cells on a per-microgram basis. This enables saRNA to be delivered at lower overall concentrations than other RNA-based vaccine platforms, which may offer reduced reactogenicity while improving immunogenicity. Additionally, saRNA vaccines have the same benefits as mRNA vaccines: they can be produced at scale in small bioreactors capable of producing hundreds of millions of doses; they can be quickly adapted for emerging viral variants; and the platform can be applied to novel viruses.
ACCELERATING LAAB DISCOVERY
Prophylaxis with mAbs has a demonstrated history of curbing infectious diseases2. More recently, innovations in protein engineering led to the discovery of the YTE modification — a set of three amino-acid changes to the antibody fragment crystallizable (Fc) ‘tail’ region — which increases an antibody’s half-life in serum by threefold to fourfold. These changes allow LAABs to be derived from naturally produced mAbs and enable long-term protection against infectious disease2. This approach is being used to develop LAABs for COVID-19 and other pathogens including respiratory syncytial virus (RSV)2,5. LAABs are critical for providing passive immunity to protect against viral disease in vulnerable individuals. By mimicking neutralizing antibodies induced by infection or vaccination, LAABs can restrict the ability of a virus to engage with host-cell receptors and prevent host-cell entry2. LAABs can be used as prophylaxes to prevent disease or as early treatments following infection2,6.
In line with the P3 goal of producing an antibody to stop a novel virus in less than 60 days, we challenged ourselves to condense our antibody discovery timeline from years to weeks. We and others developed new technologies from flow-cytometry-based single-cell sorting of antigenspecific B cells to single-cell DNA sequencing that revolutionized how we discovered ultra-potent neutralizing antibodies. These technologies were employed to discover new therapeutic antibodies in the fight against COVID-196,7.
LAABs are produced in largescale, batch-fed, 15,000-liter bioreactors using immortalized cells. AstraZeneca is developing perfusion-based manufacturing capabilities to continuously produce LAABs more efficiently at an even greater scale. Perfusion-based approaches use a cell-retention device to achieve and maintain high cellculture densities, coupled with continuous media exchange to remove waste products and old culture media, and to capture the desired antibody products. As a result, yields are higher compared with traditional batch and fedbatch cell-culture processing approaches8. Collectively, these technologies will allow us to quickly identify and rapidly produce LAABs for novel emerging pathogens.
ASTRAZENECA’S ONGOING COMMITMENT TO VACCINES AND IMMUNE THERAPIES
AstraZeneca supports broad and equitable vaccine access. With a commitment to increase investment in vaccines and immune therapies, AstraZeneca is looking to identify global needs for vaccines against other infectious agents including influenza — a leading cause of respiratory illness and death, and a serious future pandemic threat — and other NIAID P3 priority and prototype pathogens with future pandemic potential. Until we can come up with countermeasures to protect everyone, including the most vulnerable, our work will not be done.
ACKNOWLEDGEMENT
Medical writing support was provided by Craig O’Hare of Ashfield MedComms, an Inizio company, in accordance with Good Publication Practice 2022 guidelines and funded by AstraZeneca.
AUTHORS
Tonya L. Villafana, Vice President, Global Franchise Head, Infection, Vaccines & Immune Therapies, BioPharmaceuticals R&D
Mark T. Esser, Vice President, Vaccines & Immune Therapies, BioPharmaceuticals R&D
REFERENCES
- Watson, O.J. et al. Lancet Infect. Dis. 22, 1293–1302 (2022).
- Pantaleo, G. et al. Nat. Rev. Drug Discov. 21, 676–696 (2022).
- Cristina Cassetti, M. et al. J. Infect. Dis. doi:10.1093/infdis/jiac296 (2022).
- Bloom, K. et al. Gene Ther. 28, 117–129 (2021).
- Zhu, Q. et al. Sci. Transl. Med. 9, eaaj1928 (2017).
- Zost, S.J. et al. Nature. 584, 443–449 (2020).
- Loo, Y.M. et al. Sci. Trans. Med. 14, eabl8124 (2022).
- Bielser, J.M. et al. Biotechnol. Adv. 36, 1328–1340 (2018).

