When human bones suffer small defects such as fractures, they usually heal spontaneously, with the body restoring lost tissue. But when there are large defects, such as substantial bone loss resulting from removal of tumors by surgery, physical trauma, tooth extraction, gum disease or inflammation around dental implants, the bone is unable to renew itself.
Now, an innovative new technology developed at Tel Aviv University will reportedly make possible the regeneration of bone to correct major defects by means of a special hydrogel (a three-dimensional network of polymers that can swell in water and hold a large amount of it while maintaining the structure). Following successful tests in an animal model, the researchers now plan to move forward to clinical trials.
The groundbreaking study was conducted by experts from TAU’s Goldschleger School of Dental Medicine, led by Prof. Lihi Adler-Abramovich and Dr. Michal Halperin-Sternfeld, in collaboration with Prof. Itzhak Binderman, Dr. Rachel Sarig, Dr. Moran Aviv and researchers from the University of Michigan in Ann Arbor. The paper was published in the Journal of Clinical Periodontology under the title “Immunomodulatory fibrous hyaluronic acid-Fmoc-diphenylalanine-based hydrogel induces bone regeneration.”
How did Israeli researchers induce bone regeneration to fix defects?
The team developed a hydrogel matrix of bones, stimulating bone growth and reactivating the immune system to accelerate the healing process.
The extracellular matrix is the substance surrounding our cells, providing them with structural support. Every type of tissue in our body has a specific extracellular matrix consisting of suitable substances with the right mechanical properties. The new hydrogel has a filament structure that mimics that of the natural bone's extracellular matrix. It is also rigid, thus enabling the patient’s cells to differentiate into bone-forming cells.
“As can be expected, the extracellular matrix of our bones is quite rigid,” noted Adler-Abramovich. “In our study, we produced a hydrogel that mimics this specific matrix in both chemical and physical properties. At the nanometric level, the cell can attach itself to the gel, gaining structural support and receiving relevant mechanical signals from the fibers.
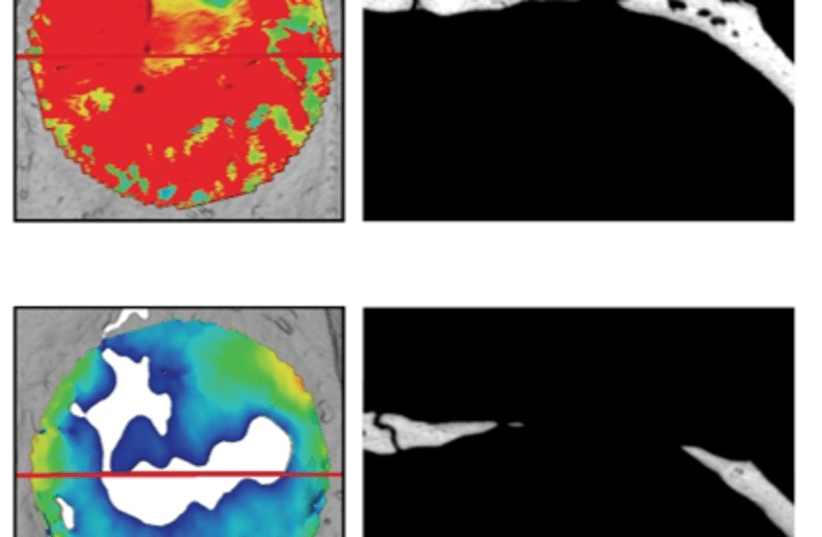
At first, to test these properties, we grew cells in a 3D model of the gel," she said. "Then we examined the impact of the hydrogel on model animals with large bone defects that could not heal spontaneously. We monitored them for two months with various methods. To our delight, the bone defects were fully corrected through regeneration, with the bones regaining their original thickness, and generating new blood vessels.”
“When we lose teeth due to extensive damage or bacterial infections, the standard treatment is dental implants, but these must be anchored in a sufficient amount of bone, and when bone loss is too substantial, physicians implant additional bone from a healthy part of the body – a complex medical procedure.
Another option is adding bone substitutes from either human or animal sources, but these might generate an immune response. I hope that in the future the hydrogel we have developed will enable faster, safer, and simpler bone restoration.”
Prof. Lihi Adler-Abramovich
The new gel has extensive clinical applications in both orthopedic and dental medicine, the researcher suggested. “When we lose teeth due to extensive damage or bacterial infections, the standard treatment is dental implants, but these must be anchored in a sufficient amount of bone – and when bone loss is too substantial, physicians implant additional bone from a healthy part of the body: a complex medical procedure.
"Another option is adding bone substitutes from either human or animal sources, but these might generate an immune response," Adler-Abramovich said. "I hope that in the future, the hydrogel we have developed will enable faster, safer and simpler bone restoration.”