The sky is the limit with the emergence of fabricating via 3D bioprinting and laser microfabrication of structures for bio/tissue engineering.
August 16, 2022
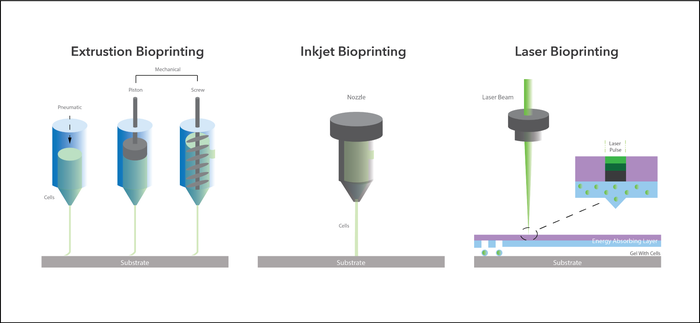
Bioprinting has been defined as the deposition of biological materials together with living cells layer-by-layer using computer-aided transfer processes. It is an additive manufacturing technology. The building of heterogeneous compositions and complex architectures has been enabled by 3D bioprinting using various techniques, including Inkjet, Extrusion, and Laser. By simultaneously printing cells and functional materials, 3D bioprinting has the capability of creating the functional properties associated with biological tissues that are needed for integration into the human body. Using such methodology allows the organizing of various cell types into desired structures, creating constructs with heterogeneous cells and vascular structures capable of recapitulating tissue features or replicating organ structures. What makes 3D bioprinting so attractive is also the possibility of high throughput and scalability while providing essential reproducibility.
Figure 1 – Current methods of Bioprinting
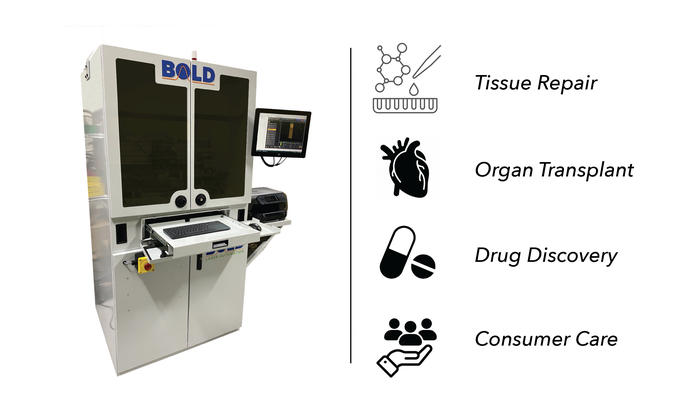
The advances in organ and micro-organ regeneration via 3D bioprinting open exciting possibilities in a myriad of different applications, not least organ transplantation so often blighted by organ shortage as donor numbers remain frustratingly low. High-throughput drug screening is also an area benefiting from advances in this technology. Cells can be assembled into models which in turn are used to make investigations into tumors. Higher cell proliferation in comparison to conventional 2D models allows for pathogenesis study as the simulation of physiological micro-environments are now part of reality. Figure 2 – High priority applications
Amongst the various bioprinting techniques available, we believe laser bioprinting, laser microfabrication, and droplet on demand of cells, offer significant advantages. Extrusion bioprinting produces filaments instead of droplets necessitating an almost fluid-like material to work successfully. In using such materials only lower resolution results tend to be possible in comparison to laser bioprinting. The use of inkjet bioprinting is stymied because of its limited capability of processing high viscous biomaterials.
The Laser Bioprinting Process
Originally used in the two-dimensional patterning of cells, laser bioprinting evolved into structuring in three dimensions. The deposition of hydrogels and cells in multiple cycles of alternating printing is an example. Hydrogels are exploited in tissue engineering because they can retain water while also maintaining their structure as they swell, composed as they are, of 3D networks of cross-linked hydrophilic polymer chains.
Typically, the pre-processing involves a range of medical imaging techniques. A tissue or organ of interest is generated using computer-aided design (CAD) underpinned by a blueprint. This blueprint is often generated using either computer tomography or magnetic resonance imaging. Focusing on a specific plane within the body, the former is used to probe deep internal structures and those structures obscured by other organs. It’s a radiologic technique that deploys a penetrating wave to generate an image by sections, stacking digitally collected “slices” into a three-dimensional picture. The blueprint allows a material and cell composition and distribution model to be arrived at.
Laser bioprinting involves the use of a pulsed laser beam (UV or IR) at µJ pulse energies, a “ribbon” or monolithic substrate with a donor transport support, and a source film that absorbs the laser energy containing the printing materials (Au is typical, however, Ti an Ag have been utilized). Forward transfer, induced by the laser, transports droplets. The high-energy transportation occurs via the production of a high-pressure bubble where a substrate collects the ejected material emanating from the source film.
Printing resolution tends to be critical and dependent on a number of parameters. The speed of the laser printing (kHz), the energy, and the pulse frequency of the laser is important but equally essential is the viscosity of the bio-inks adopted. Being nozzle-free, laser bioprinting eliminates clogging issues so often a problematic feature of both inkjet and extrusion bioprinting. Capable of very high-resolution deposits even from highly-viscose material, laser bioprinting permits single-cell manipulation and highly precise positioning of cell droplets in a controlled manner. Working with densities of up to 108 cells/mL is not an issue.
This form of droplet distribution also facilitates the use of traditional galvanometer scanning and increases laser pulse rates to affect an area which in turn enables a rapid build process. Dovetailing both direct laser microfabrication (ablation or photochemical structuring) and bioprinting, allows for a buildup process sequence that offers greater opportunity to produce more complex ECMs.
In terms of post-processing, once printed, incubation within a bioreactor tends to be undertaken to realize tissue constructs.
Precise Control and Artificial Cell Niches
Laser and Microfabrication Bioprinting (LaMB) hybrid (Patent Pending BOLD Laser Automation) process offers precise control with the capability of printing cell combinations with high viability. In having the ability to realize single spatial arrangements, it can powerfully replicate tissue and organ internal structures via cellular orienting, layering, and arranging. This allows a hybrid process to potentially generate some really niche, in fact, unique artificial cells that are in high demand within cancer and drug research circles.
Figure 3 shows the schematic layout of the BOLD LaMB process.
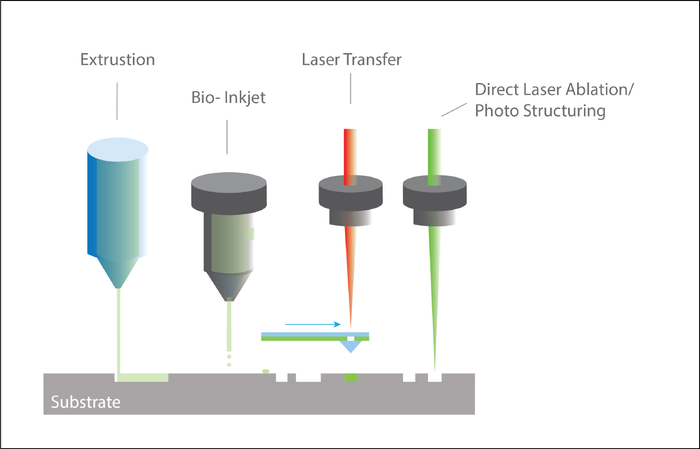
The complex structural and functional orientation of target tissues and organs also means Laser and Microfabrication Bioprinting can play a fundamental role in vitro for toxicological testing in addition to the highly important field of drug screening.
Amongst other tissue-engineering, Laser and Microfabrication Bioprinting can realize enhanced bone formation, specifically for large bone defects via improved vascularization or better network construction of blood vessels and tissue integration.
Laser and Microfabrication Bioprinting can potentially generate printed skin grafts that can be implanted into skin wounds because within the fabrication schema, accurate construction can be enabled with biomolecules and the spatial arrangement of native skin. This makes it possible to form dense, stratified tissue with complete epidermis and fully functional vascularisation. Morphologically and biologically realistic epidermis and dermis layers can be realized by engineering keratinocytes and fibroblast cells respectively. The printing of living skin with blood vessels, in all high probability, will continue to grow as a field of Bioprinting and with the scaling of a Laser and Microfabrication Bioprinting, a technique to underpin it.
The Industrial Future for Tissue Bio-fabrication using Laser and Microfabrication Bioprinting
In all likelihood, hybrid techniques, such as, Laser and Microfabrication Bioprinting will continue to diverge from traditional scaffold fabrication, inducing tissue and organ regeneration beyond the scope of previous techniques. The combination of intricate structural scaffold printing with the construction of architectures that don’t compromise cellular viability or undermine material integrity is key to facilitating cell-scaffold interaction and subsequently the realization of complex microarchitecture. Such complex microarchitectures are likely to be integral to several future directions of travel for tissue bio-fabrication on an industrial scale. Support from existing pharmaceutical contract manufacturing dedicated to specific market growth segments is possible in the mix. In addition to the bioprinting of spinal cord sections, printable bio-, and the transformation of growing cells that turn into tissue, we highlight three other areas where Laser and Microfabrication Bioprinting could be integral to their advancement.
In terms of body components, Laser and Microfabrication Bioprinting parts of the human heart and cardiac tissue engineering is an area that we can only see expanding. Valves have already been successfully bio-printed but the medical science community predicts that the arrival of a bioprinted heart suitable for transplantation as many as 15 years away. Whereas the mimicking of the heterogeneous structure of aortic valve root walls is possible via bioprinting (by encapsulating aortic valve interstitial cells within printed hydrogels) many challenges remain.
In terms of driving new printable biomaterials, any material with a proven ability to support continued cell life while retaining potency will always be of interest. The field remains receptive to both synthetic and natural polymers both biocompatible and biodegradable with the necessary mechanical strength for printing. Laser and Microfabrication Bioprinting will be in that space. Fabricating heterogeneous tissues, and depositing both biopolymer and thermoplastic polymer would expand the use and range of hydrogels, given that most biopolymer hydrogels in isolation tend to be mechanically weak. Ceramic or glass particles, mixed with hydrogels and incorporated into 3D bio-printed scaffolds, can reinforce the mechanical strength and improve the bioactivity of polymer matrixes with enhanced cell proliferation. Upon exposure to ultraviolet light, coupled with 2D and 3D scanning techniques, hyaluronic acid, for example, possesses adequate mechanical strength to realize the printing of hard tissues.
In terms of processing, the vascularisation of thick tissue constructs remains a fundamental challenge. Bioprinting 3D microchannels is one approach with promise. Created within hydrogels, hollow channels could bring adequate vascular perfusion to fruition. Facilitating the transportation of nutrients while allowing the removal of waste would greatly enhance cellular viability in addition to achieving adequate structure definition and cell density. Replicating a complex hierarchical structure of native 3D vasculatures could be the role of Laser and Microfabrication Bioprinting.
The Future
Tools offering greater opportunities for through processing will underpin the future of hybrid bioprinting. Technological process advancements in the coming decade will require the application of techniques capable of scaling up to a degree that allows the support of accessible testing regimens. Whether it’s new drugs or therapeutics undertaken in a safely controlled manner that will, one day, relieve people from disabling injuries and disease, we firmly believe that our hybrid processing tool, a product that utilizes our mix of Laser and Microfabrication Bioprinting (LaMB) will be instrumental in taking bio-fabrication to the next level.
About the Author(s)
You May Also Like


