Dopamine
One Way the Brain Gets Flooded With Too Much Dopamine
Genetic mechanisms may disrupt how the caudate nucleus regulates dopamine flow.
Posted November 4, 2022 Reviewed by Ekua Hagan
Key points
- The dopamine hypothesis of schizophrenia (SCZ) posits that having too much dopamine in the brain causes SCZ symptoms such as psychosis.
- New post-mortem research on hundreds of human brains identifies a causative link between schizophrenia and unregulated dopamine flow.
- In patients with schizophrenia, gene expression in the striatum's caudate nucleus disrupts D2-autoreceptors' ability to regulate dopamine flow.
Since the 1950s, scientists have been trying to figure out why having too much dopamine in the brain is associated with schizophrenia. The dopamine hypothesis of schizophrenia posits that in people with this brain illness, the flow of dopamine along various pathways becomes dysregulated in ways that flood the brain with excessive amounts of this neurotransmitter.
It's well-established that dopamine pathways are involved in the pathophysiology of schizophrenia and psychosis. However, researchers are still trying to figure out exactly how and why dopamine pathways get flooded with too much dopamine.
D2-Autoreceptors Slow the Flow of Dopamine Along Specific Pathways
There are four major dopamine pathways in the human brain. Of these four dopaminergic pathways, the nigrostriatal and mesolimbic dopamine systems—which play a key role in reward-seeking behavior, motivation, motor control, and addiction—flow through the caudate nucleus region of the basal ganglia's striatum.
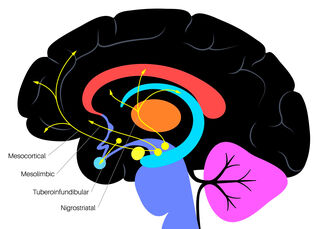
The regulation of striatal dopamine release in the caudate nucleus is controlled by a subset of inhibitory dopamine receptors called D2-autoreceptors. When these autoreceptors function properly, they maintain a "Goldilocks zone" of just enough dopamine but not too much.
In healthy individuals not experiencing schizophrenic or psychotic symptoms, these presynaptic autoreceptors act like gatekeepers that slow the flow of dopamine. But if autoreceptors are compromised and can't inhibit the amount of dopamine released from presynaptic dopaminergic neurons, the brain gets flooded with too much dopamine.
Ideally, autoreceptors in the caudate nucleus inhibit dopamine flow in ways that help maintain a dynamic equilibrium between its release and reuptake via dopaminergic pathways that travel to other parts of the brain. Antipsychotic medications turbocharge the ability of autoreceptors to inhibit the synthesis and release of dopamine, which prevents it from flooding the brain.
The molecular mechanisms that facilitate the inhibition of dopamine release by D2-autoreceptors and precisely how antipsychotics bolster autoreceptors' ability to regulate dopamine flow aren't fully understood. However, pioneering post-mortem brain research sheds light on how genetic mechanisms may alter the ability of autoreceptors in the caudate nucleus to slow dopamine's flow.
A Vexing Riddle: What's the Link Between Dopamine and Schizophrenia?
A new study (Benjamin et al., 2022) on the dopamine hypothesis of schizophrenia by researchers at the Lieber Institute for Brain Development (LIBD) gives us fresh clues about how gene expression in the caudate nucleus may affect autoreceptors' ability to inhibit the flow of dopamine in people with schizophrenia.
The findings of this recent post-mortem analysis of the genetic and transcriptional landscape of the striatum's caudate nucleus in 443 people (245 neurotypical individuals, 154 with schizophrenia, 44 with bipolar disorder) were published on November 1 in the peer-reviewed journal Nature Neuroscience.
"Most studies of gene expression in the brains of individuals with schizophrenia have focused on cortical regions, but subcortical nuclei such as the striatum are prominently implicated in the disease, and current antipsychotic drugs target the striatum's dense dopaminergic innervation," the authors explain. The researchers note that they "identified many genes associated with schizophrenia risk" and found that antipsychotic medications have an "extensive influence on caudate gene expression."
The LIBD researchers identified specific genetic mechanisms that disrupt D2-autoreceptors' ability to regulate dopamine flow in individuals with schizophrenia. "If autoreceptors are compromised, the flow of dopamine within the brain is poorly controlled, and too much dopamine flows for too long," Benjamin et al. explain.
"Until now, scientists have been unable to decipher whether the dopamine link was a causative factor or solely a way to treat schizophrenia," senior author Daniel Weinberger said in a November 2022 news release. "We have the first evidence that dopamine is a causative factor in schizophrenia."
"One of the major side effects of the drugs used to treat schizophrenia is lack of pleasure and joy," co-author Jennifer Erwin added. "In theory, if we could target the dopamine receptor specifically with drugs, that could be a new strategy for treatment that would not limit a patient's joy as much."
In 1976, Solomon Snyder, a neuroscientist from Johns Hopkins University School of Medicine, unearthed how antipsychotic drugs help patients with schizophrenia by reducing dopamine levels in the brain. In a November 2022 statement, Snyder, who wasn't involved in Benjamin et al.'s recent study, hailed the latest LIBD findings as a "breakthrough many decades in the making."
"For decades, people have debated the dopamine connection to schizophrenia," Snyder said. "They used to say, 'Well, this is interesting to speculate about, but there's no solid evidence.' But now that we have much more rigorous data available, we keep coming back to the same story. You don't have to call it a hypothesis anymore."
Autoreceptors in the Caudate Nucleus Could Be a New Therapeutic Target
The latest (2022) study by Benjamin et al. provides fresh insights into genetic mechanisms that disrupt the ability of D2-autoreceptors in the caudate nucleus to inhibit the flow of dopamine. Identifying these autoreceptors' vital role in preventing the brain from getting flooded with too much dopamine could lead to new psychopharmacology targets in the caudate nucleus.
References
Kynon J. M. Benjamin, Qiang Chen, Andrew E. Jaffe, Joshua M. Stolz, Leonardo Collado-Torres, Louise A. Huuki-Myers, Emily E. Burke, Ria Arora, Arthur S. Feltrin, André Rocha Barbosa, Eugenia Radulescu, Giulio Pergola, Joo Heon Shin, William S. Ulrich, Amy Deep-Soboslay, Ran Tao, the BrainSeq Consortium, Thomas M. Hyde, Joel E. Kleinman, Jennifer A. Erwin, Daniel R. Weinberger & Apuã C. M. Paquola. "Analysis of the Caudate Nucleus Transcriptome in Individuals With Schizophrenia Highlights Effects of Antipsychotics and New Risk Genes." Nature Neuroscience (First published: November 01, 2022) DOI: 10.1038/s41593-022-01182-7
Christopher P. Ford. "The Role of D2-Autoreceptors in Regulating Dopamine Neuron Activity and Transmission." Neuroscience (First published: January 23, 2014) DOI: 10.1016/j.neuroscience.2014.01.025
Ian Creese, David R. Burtand, Solomon H. Snyder. "Dopamine Receptor Binding Predicts Clinical and Pharmacological Potencies of Antischizophrenic Drugs." Science (First published: April 30, 1976) DOI: 10.1126/science.3854




