Human beings are complex. Unfortunately, our current approaches to health and medicine are based on simplistic models that fail to take into account the intricate relationships between body, mind, and our social and physical environments. Our health-care system rarely considers that humans are products of both nature and nurture and that our minds influence our bodies, and vice versa. We give short shrift to diversity in both ethnic and social backgrounds, even though it’s well known that both play a major role in health.
Worse, we generally don’t seek information about our health and well-being until we develop symptoms, by which time it may be too late to reverse the transition from wellness to disease.
More than a decade ago, a group of us at the Institute for Systems Biology in Seattle began to think about how the practice of medicine could focus more on wellness than disease. Rather than waiting until people get sick, what if we found ways of helping people stay healthy?
Part of what inspired this question was the tremendous amount of data becoming available through advances in technology, such as low-cost genetic sequencing. These tools emerged from “multiomics”, which collectively refers to new data-driven fields of inquiry into the body’s many biological constituents. The “omics” in the name comes from the common methods used to analyze four different types of constituents: proteins (proteomics), metabolites (metabolomics), genes (genomics) and gut bacteria (microbiomics).
Since then, we’ve developed these ideas and honed them through research with thousands of people. We’ve found that the copious “omic” data becoming available gives clinicians an opportunity not only to diagnose disease, but also to take the measure of an individual’s state of wellness. We are developing ways of using this data to identify early signs that things are going off track—in other words, when a person begins the transition from wellness to illness. And we are fashioning interventions that get them back on track before and during this disease transition, sometimes avoiding disease entirely.
These interventions need not be drastic or invasive. Even small changes to diet and exercise or targeted supplements could potentially head off obesity, diabetes, heart disease and other ailments before they have a chance to develop. The key is to intervene early, before the onset of disease. By informing these interventions with behavioral science, we’ve been able to deliver interventions that people actively incorporate into their daily lives, often with dramatic results.
We are now focused on filling the research gaps in our understanding of disease transitions and how to prevent them—what we have come to call “scientific wellness.” At the same time, we need to remove many practical roadblocks to realize a truly preventive and personalized approach to medicine. But nevertheless, our work has demonstrated unequivocally that this approach could vastly improve health-care outcomes compared with today’s disease-centric medicine. And because it is preventive, it could generate considerable cost savings.
The Pioneer 100 Pilot
Our inquiry into scientific wellness began with a pilot study, called Pioneer 100. This project was initiated by Leroy Hood, a physician-scientist who brought me in to help create the clinical protocol and oversee the study. I had conducted academic research in obesity, diabetes and women’s health and had also had executive roles at health and wellness start-ups, and I was fascinated by the potential of big data combined with behavioral science. The questions we sought to answer in this study were oriented toward proving the concept of scientific wellness. We wanted to find out if people who believe they are well have actionable opportunities to optimize wellness or prevent disease. We also wanted to know if there is enhanced value in integrating different data types to quantify wellness. Could we identify novel discoveries with multiomic data, even with a small number of participants? And how would participants respond to a scientific wellness intervention?
We recruited 108 healthy volunteers to participate in a nine-month study seeking to both quantify and optimize wellness. The first thing we did was gather baseline data, which we would later use to develop action plans, perform scientific research and assess the program’s success. We collected data on a variety of health measures. Rather than rely on surveys of food intake, which are notoriously unreliable, we measured nutritional biomarkers—molecules in the blood that give a more accurate indication of what an individual is eating. (In general, in this study we relied on measurements of biomarkers, which could be made with precision, rather than self-reported disease or conditions.) We had the volunteers wear Fitbits that monitored their physical activity and sleep. We also took a variety of lifestyle and psychological assessments, which we shared with behavioral coaches, who helped the volunteers develop and implement personalized action plans to reduce risk factors. And we gathered multiomic data—we sequenced their genomes, sampled their gut bacteria, and measured their metabolites and proteins. This gave us a total of six types of data which we would be able to mine for novel insights: clinical, lifestyle, genomics, proteomics, metabolomics and gut microbiomics.
Once we had reviewed the baseline data, we drafted for each participant an individual lifestyle intervention plan, which included a range of actionable possibilities that would ameliorate or potentially reverse any risk factors for disease. For instance, someone with high cortisol would be supported in developing a daily stress management practice and given a series of exercises to identify and address their stressors. Or an individual who was 20 pounds overweight and had been trying unsuccessfully to lose weight on a low-carbohydrate diet would be educated in a dietary pattern that aligned with their weight-related genetic profile. In some cases, such as for people with low levels of vitamin D or omega-3 fatty acid levels, we would recommend a dietary supplement. Each participant then began a series of health coaching sessions with a registered dietitian, who provided behavioral and nutritional support. The blood markers, gut microbiome and lifestyle data were assessed every three months so we could determine the impact of the lifestyle coaching on wellness.
The results of the study were compelling. At baseline, all participants had multiple actionable possibilities. Some had out-of-range results on standard clinical tests, such as high cholesterol or glucose levels. Many had nutritional deficiencies, genetic risk factors for various conditions, or gut dysbiosis (an imbalance of gut microflora).
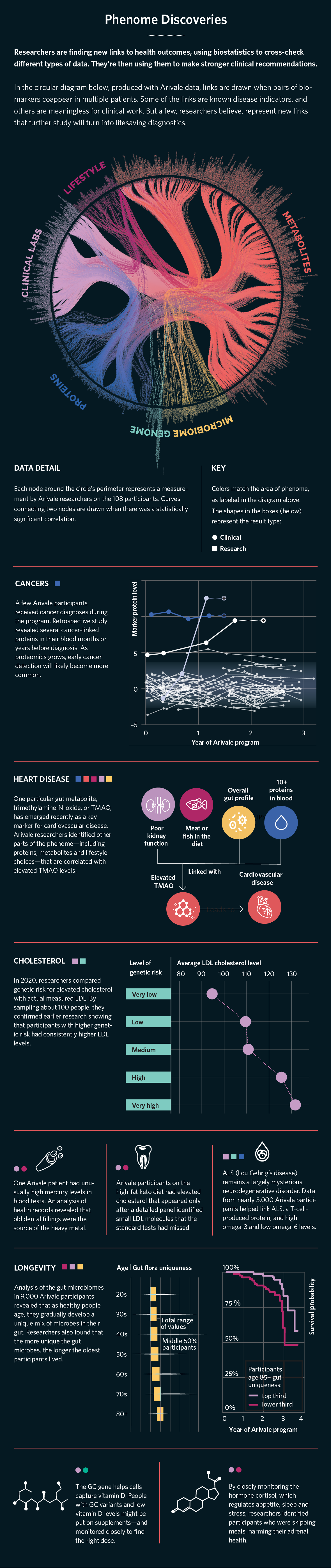
We found more than 14,000 statistically significant correlations between our six data types (see "Phenome Discoveries" on p. 26). Many of these correlations led to greater personalization of recommendations, such as the ability to use genetics to better optimize dietary tips. And several generated novel discoveries that could be subsequently tested in clinical trials. For example, we found that a certain liver enzyme (gamma-glutamyl transferase, or GGT) was associated with multiple risk factors for cardiometabolic syndrome, a group of metabolic dysfunctions that raise the risk of coronary heart disease, stroke, and diabetes. We also found that higher gut microbiome diversity was associated with lower inflammatory proteins.
We also created polygenic risk scores (which examine the cumulative total of hundreds or thousands of single mutations related to a particular disease) and observed alterations in blood markers associated with these scores that could be early indicators of disease. For example, we found that a high polygenic risk score for inflammatory bowel disease was associated with lower levels of cystine, an amino acid metabolite, in the blood, and that a high polygenic risk score for bladder cancer was associated with higher levels of a metabolite of caffeine. Future studies could be designed to examine the role of these biomarkers in development of disease in people who are at risk, potentially leading to future treatments or preventive strategies. More important, we found that behavioral coaching was effective in driving significant clinical improvements across various integrated biomarkers. Study participants were highly enthusiastic about this approach.
Broadening Our Reach
On the basis of Pioneer 100’s success, in 2015 we launched Arivale, a genetic testing and personal health coaching company. One goal was to bring the benefits of scientific wellness to large numbers of consumers. We also wanted to create a unique database of multiomic data from individuals, collected over time, that could be mined to generate scientific discoveries and novel clinical treatments. Over four years, Arivale provided multiomic data and behavioral coaching to more than 5,000 people, most of whom enrolled for two to three years. This gave us a deep longitudinal data set and opportunities to see significant improvements in wellness.
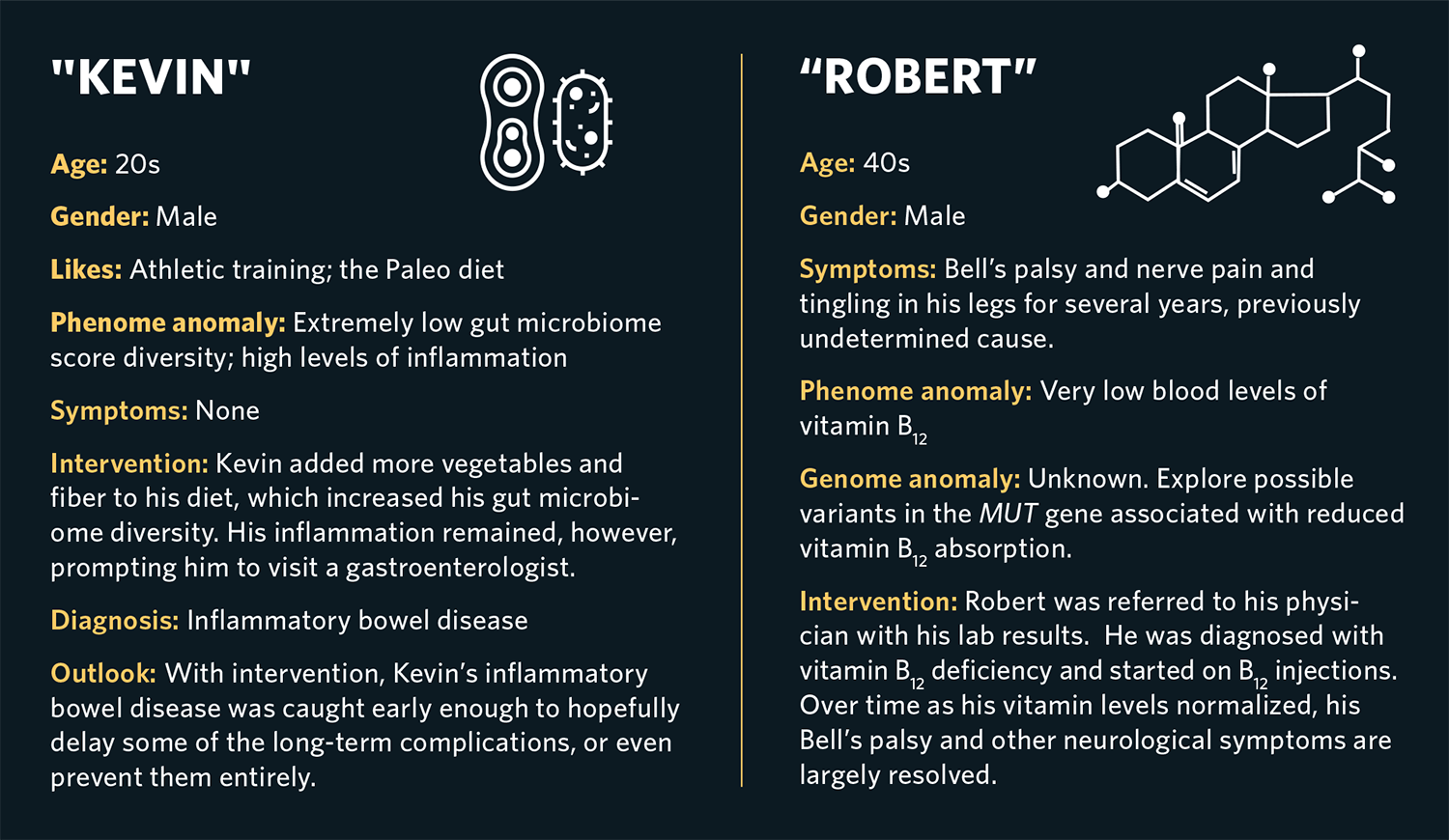
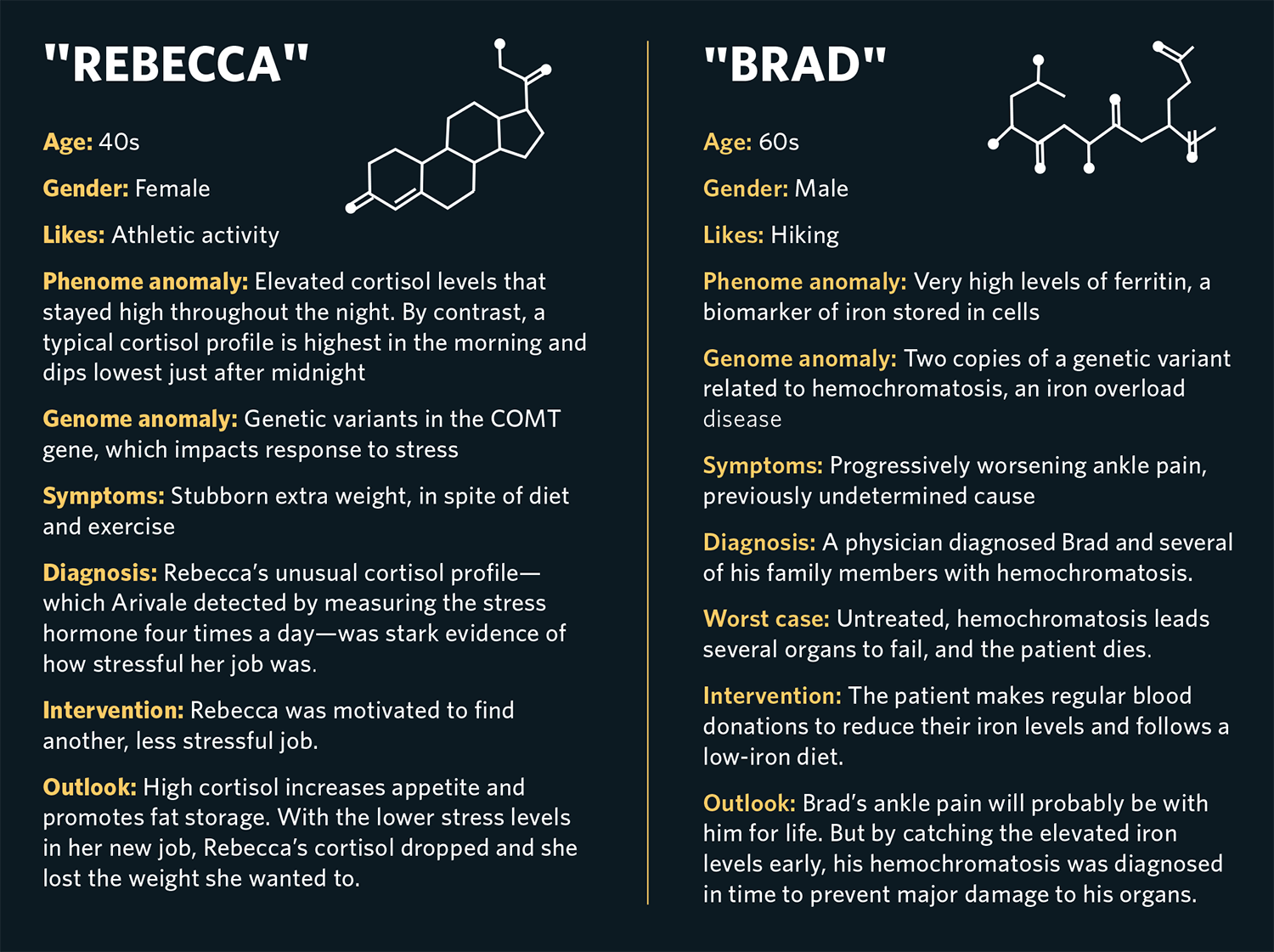
Arivale’s scientific wellness approach had a substantial impact on the lives of its participants. Brad (not his real name), a man in his 60s who was an avid hiker, suffered from progressively worsening pain in his ankle that his physicians were unable to diagnose. Baseline multiomic data showed that he had very high blood levels of ferritin, a biomarker of iron stored in his cells, and two copies of a genetic variant related to hemochromatosis, an iron overload disease and a common genetic disorder in people of European descent. It turns out that one of the symptoms of this disease is joint pain. Untreated, hemochromatosis ultimately leads to multiorgan failure and death. We referred Brad to his physician, who ultimately diagnosed him and several members of his family with the disorder.
The treatment for hemochromatosis is straightforward: regular blood donation until iron levels return to normal. But since clinicians do not routinely measure ferritin levels or genetic risk, they typically don’t discover hemochromatosis until it is significantly more advanced. Although early diagnosis did not reverse Brad’s ankle pain, it most likely avoided major damage to his heart, liver and other organs down the road.
Similarly, baseline testing found that Kevin (also a pseudonym), an apparently healthy athlete and trainer in his late 20s, had high levels of inflammation markers and an extremely low gut microbiome diversity score. His lifestyle data showed that he followed a Paleo-type diet consisting mostly of meat and dairy products with few vegetables. Since low intake of fiber is associated with poor gut microbiome diversity, we coached Kevin on how to increase his intake of fiber-rich foods, such as vegetables, fruits and intact whole grains. After six months, his gut microbiome had improved but was still not normal. Spurred by his ongoing elevated inflammatory profile, Kevin followed up with a gastroenterologist who diagnosed inflammatory bowel disease. Since this was caught much earlier than it normally would have been, his doctor was able to initiate treatment and hopefully delay or prevent some of the long-term complications. Furthermore, what Kevin learned about his digestive issues and gut microbiome pattern helped him choose a diet that was much healthier for his unique biology than the one he’d originally followed.
From the standpoint of scientific discovery, the data generated through scientific wellness interventions has also proven to be of value. Our work formed the basis of published papers on topics including predictive risk factors for metastatic cancers, gut microbiome in aging and obesity and the impact of a scientific wellness intervention in patients with early-stage cognitive decline.
Conventional Wellness vs. Scientific Wellness
Most general wellness programs tend to focus on nutrition, physical activity, stress and sleep, which are all proven methods to improve health and prevent disease. The difference with scientific wellness is that it approaches these same interventions with a rigorous, data-driven mindset.
For example, our clients at Arivale often had been taking dietary supplements for nutrients for which their blood levels were perfectly normal, or even high. Based on scientific wellness data, they could stop taking the supplements they didn’t need. Conversely, we commonly found certain nutritional deficiencies, such as vitamin D, omega-3 fatty acids and ferritin, which were easily treated.
For each nutrient, there are genetic markers that indicate how well a person is likely to absorb or metabolize the nutrient. For example, individuals with a specific variant of the GC gene, which codes for a vitamin D-binding protein, typically have lower blood levels of vitamin D and may need to take higher doses of supplemental vitamin D to get their blood levels up to normal. This sort of tailoring of nutritional recommendations cannot be done without both blood and genetic measurements.
Another area where the data-driven approach made a difference was weight loss. Many of our clients came into the scientific wellness program following a low-carbohydrate diet, which was popular at the time, and yet struggled to lose weight. Some of them turned out to have genetic variants that correlate with greater success at weight loss on a low-fat diet. After a dietitian coach shared this information with our clients, many discovered that reducing fat and increasing high-fiber, complex carbohydrates resulted in easier, more sustainable weight loss.
For exercise, genetic data can yield information on risk of injury and speed of recovery that is useful for developing tailored exercise plans, especially for athletes or those engaging in competitive sports. The clinical biomarkers of inflammation could also tell us if someone was overexercising, resulting in chronic inflammation, a risk factor for cardiovascular disease.
Stress has a big impact on health, but figuring out how much stress an individual is truly experiencing is difficult. We found that measuring levels of cortisol, a “fight-or-flight” hormone, four times throughout the day—from morning to late evening—yielded useful insight about how our clients were handling stress in their lives. Cortisol, like many hormones, follows a specific diurnal pattern: It is highest in the early morning and then declines throughout the day and evening, reaching a low around midnight, where it remains for several hours before starting to rise again. When an individual experiences chronic stress, their cortisol curve may flatten—in other words, it may fail to decline much during the day, or not rise to normal levels in the morning. Poor sleep might appear as an unusually high morning cortisol level, and skipping lunch can lead to a high late-afternoon cortisol level, both of which create unusual patterns in the daily cortisol curve. For instance, Rebecca (not her real name), an athletic woman who struggled to lose excess weight, had a stressful job. Her overall cortisol measured very high, and her daily curve was flattened relative to normal. When she realized the impact her work was having on her cortisol levels, she was motivated to find a different and less stressful job. Since high cortisol promotes fat storage and increases appetite, the lower cortisol levels allowed her to lose excess weight.
Behavioral Science in Scientific Wellness
Data alone does not change human behavior. To get otherwise healthy people to make changes in their lives, a wellness program cannot rely solely on its data. For this reason, behavioral science and coaching were key components of our scientific wellness program.
Coaching provides motivation, accountability, health education and cognitive restructuring tools to support people in setting achievable goals in service of a larger, personal vision. The vision starts with a person’s answer to the question, “Why do you want to be well?” Flowing from that vision and from the multiomic data are a series of short-term, specific and actionable goals: swap out two sodas per day with sparkling water, walk for 15 minutes after dinner three times this week, download a mindfulness app and use it every evening before bed. As each goal is reached, new goals are added to bring the person toward their desired state of wellness.

Mindset—what we believe about our health and our healthy behaviors—is an important element of wellness. People who believe they are healthier than others of their same age—regardless of whether they actually are—tend to live longer and develop fewer diseases over time. This placebo effect also applies to lifestyle changes. A Stanford University study showed that when hotel housekeepers were told that the physical activity they engaged in during their jobs met the government guidelines for daily exercise, they tended to lose weight and their blood pressure decreased compared to a control group, even though they made no measurable changes to their daily activity or diet.
The nocebo effect is another important aspect of mindset. The nocebo effect is a situation in which patients develop side effects or symptoms from a drug or therapy just because they believe the side effects will occur. The classic example of nocebo is when someone is given an allergy test with a substance they believe causes skin reactions and then develops a rash and welt at the site—but the substance was in fact plain water.
Nocebo responses are common in nutrition because the gut is highly sensitive to emotional and cognitive patterns. For instance, celiac disease is an autoimmune disorder that causes intolerance to the protein gluten, which is found in wheat and other grain products. However, many people without celiac disease also believe they have a sensitivity to gluten. When these individuals consume gluten, they experience a variety of symptoms, which go away when they avoid gluten. Research has shown that mindset often drives this reaction. When some self-identified gluten-sensitive people are given gluten-free bread but are told that it has gluten, they develop the same symptoms they would have if the bread had actually contained gluten. Similarly, when given bread containing gluten but told it is gluten-free, they don’t develop the symptoms.
The key to changing behavior is to be aware of the power of mindset and its ability to help or harm in our wellness goals, and to leverage the helpful aspects of our minds to the extent possible. In health coaching, as with the medical placebo effect, we can use positive psychology strategies such as “appreciative inquiry,” which gets people to focus on their strengths and things that are working for them rather than on their perceived weaknesses or barriers to success. For example, a coach might ask someone to reflect on a time when they were successful at making a dietary change and what made it successful, or what types of exercise feel like fun and play rather than a chore. Simply reflecting on these questions often puts the person in a more positive and creative mindset that allows for better problem solving and greater success.
But that’s not to overlook the power of the data themselves. Regularly rechecking data for signs of improvement is key for motivating people to stick with—or ramp up—healthy new behaviors. For instance, improved diet and exercise show up in altered cholesterol and blood sugar levels, and improvements in stress management can lead to reduced cortisol measures. In some cases, individuals who were initially less engaged with the coaching and behavior change were motivated to improve their lifestyles only after they saw their numbers fail to change.
Scaling Scientific Wellness
Many practical challenges remain before scientific wellness can be widely adopted. Data and behavioral protocols need to be standardized. New laws and regulations will be needed to incorporate personalized wellness into standard medical practice. People will need to see for themselves and accept that this approach delivers value to them. New technologies will be important for analyzing and integrating different data types and patterns. Artificial-intelligence approaches will help scale behavioral coaching to millions of people, as this cannot feasibly be done solely with human coaches.
Research is now addressing some of the gaps in our knowledge of wellness. For example, much of what we know about genetic variants that contribute to health and disease comes from European populations and may or may not apply to non-Europeans. Over the next several years, large genomic studies such as the multiethnic Population Architecture using Genomics and Epidemiology (PAGE) study, the Human Heredity & Health in Africa (H3Africa) Initiative, and studies in Asian populations expanding on the 2009 HUGO Pan-Asian SNP Consortium are likely to give us the data to begin to incorporate genomic information from non-Eurocentric racial and ethnic groups.
We also need to learn more about interventions for modifying the gut microbiome. Lifestyle changes (diet, exercise, stress) or probiotic supplements are the most common approaches. For instance, increasing dietary intake of high-fiber foods generally improves gut microbiome diversity and overall health at a population level. Only recently have we begun to get enough information to offer personalized recommendations. For instance, we now know that a metabolite called TMAO (trimethylamine-N-oxide) is associated with heart disease. Specific bacteria in the gut produce the precursor to TMAO when red meat and certain other foods are consumed. Knowing whether an individual has these bacteria allows for personalized recommendations about how much red meat intake is optimal for heart health.
Another important emerging area ties the gut microbiome to blood glucose levels and diabetes risk. A recent study from the Weizmann Institute in Israel compared the effects of a diet specifically personalized to the individual’s gut microbiome vs. a healthy Mediterranean diet in people newly diagnosed with type 2 diabetes. The microbiome-personalized diet resulted in significantly lower glucose levels and diabetes remission in 61 percent of participants. This shows that we can use specific microbiome data to design an individualized diet, and that doing so results in better outcomes than a plant-rich Mediterranean diet.
Wearable devices will likely play a significant role in scientific wellness. Nearly 900 different devices and wearable sensors are now on the market, including smartwatches that measure activity and heart rate as well as devices that measure sleep, autonomic nervous system function, blood oxygen and glucose. Today this technology is underused for wellness—we seem to have more comprehensive and accurate sensors for our cars than we do for our bodies. However, considerable investment and ongoing research in both academia and industry are addressing this deficiency. New technologies are being developed to automatically measure dietary intake and the impact of diet on key health markers, mental health applications that detect and offer behavioral interventions for anxiety or depression, and women’s health-related wearables that track the menstrual cycle, fertility and pregnancy-related factors.
Metabolomics and proteomics are still lagging when it comes to clinical applications to scientific wellness interventions. Still, both types of data are providing tremendous scientific insight. For example, a group from Stanford University recently described a metabolite, Lac-Phe (N-lactoyl-phenylalanine), that is produced during exercise and acts to suppress appetite. In lab animals, administration of Lac-Phe suppressed food intake by 50 percent and caused weight loss. However, it will be many years before human safety and efficacy data suffice for this to be used in a clinical setting. In proteomics, clinical and translational findings have emerged primarily in clinical medicine and oncology rather than wellness.
The promise of scientific wellness in terms of opportunities to define and optimize individual healthfulness and generate important scientific discoveries is clear. Unfortunately, consumers don’t seem to be willing to pay for data-driven wellness and prevention. Until we can get consumers and payers to grasp the value of identifying risk factors and early transitions to disease when they can still be reversed, adoption of scientific wellness will be slow. Government can help by funding research studies on scientific wellness, akin to Pioneer 100 and Arivale’s research, that will convince consumers and payers of the efficacy of scientific wellness.
Since scientific wellness is foundational to precision medicine, we won’t fully realize the benefits and opportunities of one without the other. With some creative solutions to the barriers of cost and mindset, however, scientific wellness can become a standard of care that allows everyone to thrive.
Jennifer C. Lovejoy is an affiliate faculty member at the Institute for Systems Biology and has a lifestyle and behavioral science consulting company Integral Science, LLC. Previously, she was the Chief Translational Science Officer at the scientific wellness company Arivale.
Find out more about Phenome Health’s efforts to transform the future of health care here. Learn more about phenomics, the new science of wellness, in other stories in this special report.



