Light can be used to control molecular handedness
In a recent study, researchers at Freie Universität Berlin, the DESY research center in Hamburg, Kiel University, and Kansas State University have shown how light can turn a planar molecule into a chiral molecule with just one particular handedness, providing a solution to the long-standing problem of absolute asymmetric synthesis. This new process could be particularly useful in chemically synthesizing compounds.
The study, carried out as part of the Collaborative Research Center 1319 Extreme Light for Sensing and Driving Molecular Chirality (ELCH), was published in Science Advances on December 7.
"Molecules that consist of four or more atoms are often chiral—which means that their atomic spatial arrangement is either left-handed or right-handed. Each chiral molecule has a twin molecule from a mirror world with the opposite handedness," explains Professor Christiane Koch from the Department of Physics at Freie Universität Berlin.
The left-handed and the right-handed versions of the molecule (also called enantiomers) are fully equivalent to each other, except that they interact very differently with their surroundings and with each other depending on the handedness. This is just like when two hands come into contact in the world of human social interactions; if two right hands are held out, then the two participants will shake hands, whereas if a right and a left hand come together, the two participants will hold hands, which is a very different gesture.
Scientists are still discovering new information about how molecules arrange themselves in a left-handed or right-handed configuration. "Biologically relevant molecules are mainly homochiral, which means that in the living cells, only one of the two possible enantiomers is present. Despite being made up of exactly the same atoms, the two mirror images of a molecule differ in many properties," explains Koch.
For example, one enantiomer might smell like oranges, while the other one smells like lemons. "Or even worse, one enantiomer will be a cure for a disease, while the other one will be a dangerous poison. Controlling the formation of one specific handedness of chiral molecules is therefore an important goal in chemical synthesis," adds Koch.
Usually, chemists rely on controlling the chirality with chiral chemicals. However, there was one alternative that had not yet been carried out: absolute asymmetric synthesis, which is the control of product chirality using only light fields. It was previously unclear as to how exactly this could be achieved.
In their work, Denis Tikhonov, Alexander Blech, Monika Leibscher, Loren Greenman, Melanie Schnell, and Christiane Koch have conceived a procedure for absolute asymmetric synthesis that can be tested in an experiment.
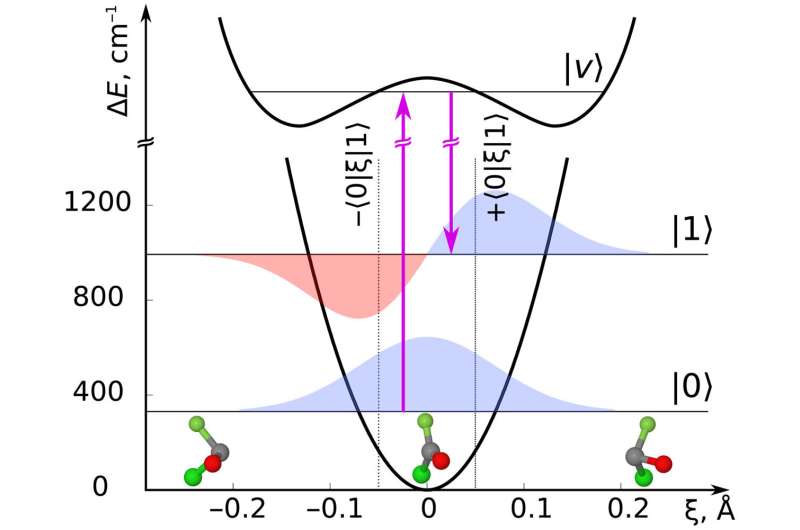
Starting from a gaseous ensemble of the planar molecule COFCl as the non-chiral reagent, the carbon molecules are made chiral by exciting vibrational motion out of the molecular plane. This preparation step is referred to as pump. As the molecule vibrates back and forth from above to below the plane, the molecular handedness changes. This change can be measured by ionizing the molecules and varying the delay in time between the pump and probe steps.
In order to be able to measure the time-dependent handedness of the whole molecular ensemble, the pump step needs to be built up by three different electric fields that have to be perpendicular to each other. Otherwise molecules at a given orientation will start in a left-handed configuration whereas molecules at another orientation will set out from a right-handed one, leaving no net signal.
The combination of electric fields that the team identified ensures that all molecules, irrespective of their orientation, start out with the same handedness. In more technical terms, the required combination of electric fields for the pump step can be realized by a left and a right circularly polarized pulse, which together induce what is known as a Raman transition, as well as a static electric field. The probe step involves ionizing the molecules. Measuring the directions into which electrons are emitted allows one to infer the molecules' handedness.
The authors argue that the proposed experiment is feasible with already existing setups, for example, by means of the circular polarization provided by FLASH at DESY.
More information: Denis S. Tikhonov et al, Pump-probe spectroscopy of chiral vibrational dynamics, Science Advances (2022). DOI: 10.1126/sciadv.ade0311
Journal information: Science Advances
Provided by Freie Universität Berlin