Philips' massive recall of sleep apnea devices highlights a big problem with at-home medical devices.
January 18, 2023
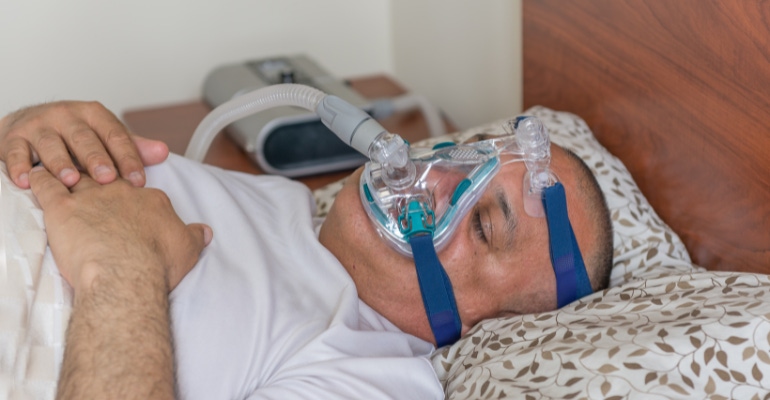
Medical devices are increasingly shifting from healthcare settings to the home, a trend that has many obvious benefits. But what happens if the medical devices you use at home get recalled?
Gaps with recalls of home-use medical devices is the most pressing health technology safety issue for 2023, according to ECRI.
The Plymouth Meeting, PA-based independent, nonprofit patient safety organization just released its annual report of health technology hazards for the year. In the report, the organization warns that recall notices for home-use products often don't reach users.
The report points to the ongoing recall of millions of continuous positive airway pressure (CPAP) and bilevel positive airway pressure (BiPAP) machines manufactured by Philips as a prime example of how real this danger is. Although Philips' recall was initiated in June 2021 and affected 5.5 million devices, several months elapsed before some patients became aware of the recall. The languge in the recall notice also confused patients about whether to continue to use the device and what actions needed to be taken, ECRI noted.
As the home healthcare trend accelerates, ECRI said it is concerned about home care patients not receiving safety notices that warn of problems with the medical devices they are using. Device manufacturers seldom have direct communication with home care patients, and healthcare providers may not proactively contact patients about recalls, the organization noted. Patients with affected medical devices may learn about a recall long after it was issued, and potentially from an unreliable source, the organization points out.
"Even if patients do receive notifications, the language may be jargon-heavy and perplexing, and patients may have difficulty determining whether their device is affected or what to do about it," said Marcus Schabacker, MD, president and CEO at ECRI. "Without clear, understandable information about a product recall, patients cannot accurately assess the health risks and may harm themselves by continuing to use an unsafe device, or by inappropriately stopping use of a device."
The annual list of health technology hazards identifies the potential sources of danger that warrant the greatest attention for the coming year, according to ECRI, and offers recommendations for reducing those risks. Since its creation in 2008, the list has supported hospitals, health systems, ambulatory surgery centers, and manufacturers in addressing risks that can impact patients and staff. New for this year, the executive brief accompanying the list now includes specific calls to action for industry.
For 2023, ECRI's report includes a series of challenges to industry, urging manufacturers of medical devices to pursue device or process improvements that could mitigate — or even eliminate — some of the hazards included on the list. With healthcare facilities understaffed and healthcare workers overstressed, it's more important than ever that technologies be designed in ways that ensure their safe use, the organization said.
"Reducing preventable harm requires more than just vigilance on the part of technology managers and device users. The medical device industry also has a role to play," said Schabacker.
On February 1, ECRI will present a live lab webcast, Home-Use Device Recalls: What You Need to Know to Mitigate Risk and Protect Patients, to provide additional information about this year's number one hazard.
The 10 topics on ECRI's 2023 hazards list are below in rank order:
Gaps in recalls for at-home medical devices cause patient confusion and harm
Growing number of defective single-use medical devices puts patients at risk
Inappropriate use of automated dispensing cabinet overrides can result in medication errors
Undetected venous needle dislodgement or access-bloodline separation during hemodialysis can lead to death
Failure to manage cybersecurity risks associated with cloud-based clinical systems can result in care disruptions
Inflatable pressure infusers can deliver fatal air emboli from IV solution bags
Confusion surrounding ventilator cleaning and disinfection requirements can lead to cross-contamination
Common misconceptions about electrosurgery can lead to serious burns
Overuse of cardiac telemetry can lead to clinician cognitive overload and missed critical events
Underreporting device-related issues may risk recurrence
About the Author(s)
You May Also Like


.png?width=300&auto=webp&quality=80&disable=upscale)

