Here are tips to help you navigate the maze of proving your groundbreaking wearable idea will work.

As we discussed a few years ago in MD-DI, innovation in consumer biometric wearables has begun to outpace that of medical monitoring technology. This progress is not only in capability but also in terms of cost, particularly regarding the sensor technology being embedded in consumer wearable devices. However, we also pointed out:
“This does not mean that consumer wearables on the market today are ready for medical use today, but rather that the technology is ready for medical validation studies.”
Since then we’ve seen a dramatic increase in validation studies using wearable devices in medical use cases. At Valencell we have been fortunate to be involved in many of these investigations, and we’ll share some of the best practices we’ve identified for successfully using wearable biometric sensors to prove medical use cases.
Start with the End in Mind
It sounds simple but it can get lost in the mix of research, validation, and product development—always keep in mind what exactly you are trying to prove in your research. In some cases, the research may be exploratory, focused on proving/disproving a hypothesis that may then lead to further research. In other cases, the end game of the research may be the development of a fieldable product, where it is important that the desired medical claims and intended use are well-defined upfront. In either case, it is important to determine the right physiological assessments that will prove outcomes and/or endpoints you’re trying to achieve. The assessments are driven by directly measured metrics from wearable devices, and those required metrics help determine which sensor modalities are required to generate those metrics. Here’s a visualization of that process:
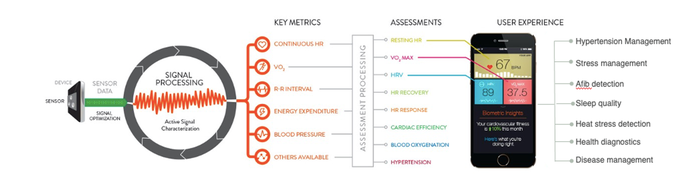
This is extremely important because the use case drives many different factors in devices used in your research:
Accuracy requirements of the devices involved
Long-term wearability required
Measurement reliability
Viable sensor modalities
Key human factors considerations
Contextual sensing required
Medical claims being made by the solution
So, let’s look at a few of those in more detail.
Just Because It Can Be Worn Doesn’t Mean It’s Truly Wearable
There are always trade-offs with biometric wearable devices and the sensor modalities involved. You can have a device that has many different sensor types (for example PPG, ECG, GSR, and EEG), has very long battery life, and takes thousands of measurements per second.
However, it’s likely to be bulky, uncomfortable, highly sensitive to sensor placement and motion artifacts (compounded by the plurality of sensor types), challenging for sensor maintenance, and not very durable (or feasible) for long-term use.
You have to find the right balance of the different factors that matter for your use case and patient experience. Typically, the levers that can be pulled and the key questions to ask are:
Wearability and comfort. How long do people need to wear the device for the study? How long do they need to wear the device to achieve the desired outcome in the real world? Does the device need to be “wear it and forget it,” or is it acceptable to ask study participants to adjust, or perform maintenance on, the wearable sensor?
Measurement autonomy. Do the measurements taken by the device need to be seamlessly and autonomously collected in the background, as people live their daily lives, or can the subject be notified to stop an activity and take a manual measurement?
Motion tolerance. Are the subjects sitting still during measurement or are they wearing the device through their daily activities?
Measurement frequency. How often is the device taking measurements?
Data communication. How often is the device communicating data and where is it sending that data?
Long-term durability. How long is the device expected to last? Electrodes, for example, have not demonstrated long term durability in wearables.
Battery life. Recharging or changing batteries has a direct impact on adherence to wearing the device. How long is long enough in battery life for your use case?
Medical Wearables Make the Most Impact in Ambulatory Medical Use Cases
Medical wearables are most relevant for medical devices that must reliably work during everyday life activities, where traditional medical benchmarks cannot function.
The definition of “regular life activities” and the required “measurement acuity” are dictated by the intended use (the use case) and the medical claims that will be made with the solution. In general, the marketplace has so far proven that it’s acceptable to trade sensor wearability over sensor acuity for ambulatory use cases.
We’ve also seen four key modes of biometric monitoring emerge in proving medical wearable use cases:
Ambulatory Monitoring. The wearable device replaces an unwieldy “medical-grade” device for ambulatory use; for example, PPG-based blood pressure monitoring replacing usage of blood pressure cuffs.
Proxy. Some wearable sensors can provide a suitable proxy for other “medical-grade” sensors; for example, PPG sensors as a proxy for EEG in neuromodulation therapy and pain management.
Augmentation. Using a wearable device to augment a pharmacotherapy or digital therapeutic to gain deeper insight into how the individual is responding to the therapy—both acutely and chronically.
Screening. The wearable device continuously screens for a potential medical condition; for example, wearables with PPG sensors used in screening for irregular heart rhythms and then prompting the individual to get an ECG reading to validate for atrial fibrillation or other arrythmias.
It Always Takes a LOT More Data than You Think—Both for Building the Solution and Then for Validating the Solution
Last but certainly not least is data collection. No matter how much data you think you need now, expect to at least double or triple it. As data science and machine-learning techniques grow in importance for utilizing wearable sensor data, the need for more data has significantly increased in nearly every use case we’ve seen. On top of that, there are limits to deep learning for addressing acute use cases, such that the data collected must be properly labeled and be aligned with reference data from a benchmark device. Whereas millions of labeled ECG waveform datasets can be obtained from dozens of universities and hospitals across the globe, the same is not true for PPG and auscultatory waveform data. These labeled datasets simply do not exist in sufficient quantity for useful deep learning approaches and thus must be created with massive data collection efforts.
In summary, we’re still in the early stages of proving new medical use cases with biometric wearables, but we hope these tips are useful in navigating the maze of proving your groundbreaking idea will work.
About the Author(s)
You May Also Like




